Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
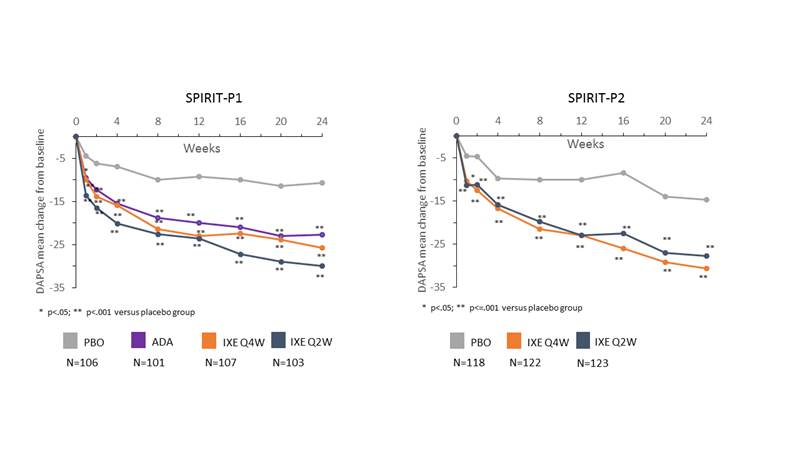
Background/Purpose: The DAPSA is a composite tool that assesses several domains of psoriatic arthritis (PsA) manifestations and was developed as a disease activity measure for clinical trials and clinical practice. Ixekizumab is a high affinity mAb against IL-17A recently approved for the treatment of PsA, and in this analysis, we evaluated the impact of ixekizumab treatment on disease activity assessed with the DAPSA through 52 weeks in patients who are biologic DMARD (bDMARD)-naïve or TNF- inadequate responders (TNF-IR).
Methods: Data from the 24-week, randomized, double-blind, placebo-controlled periods from 2 Phase 3 clinical trials (SPIRIT-P1, NCT01695239; SPIRIT-P2, NCT02349295) and their extension periods were analyzed. Patients from SPIRIT-P1 were bDMARD-naïve (N=417) and patients from SPIRIT-P2 had inadequate response or intolerance to TNF inhibitors (N=363). Patients were randomized to receive subcutaneous placebo (PBO), adalimumab (ADA, SPIRIT P1 only) or 80 mg ixekizumab every 2 (IXE Q2W) or 4 wks (IXE Q4W), after a 160 mg starting dose. The components of the DAPSA (68 tender joint counts, 66 swollen joint counts, patient assessment of pain, patient global assessment and CRP) were assessed at each visit. Comparisons versus placebo were made on changes in each component of DAPSA and the composite DAPSA score through week 24 using mixed effects model for repeated measures. Mean change values were reported through week 52 for patients initially randomized to ixekizumab treatment arms for the intent-to-treat population using last observation carried forward for imputing the missing values.
Results: Baseline DAPSA was 44.7 for bDMARD- naïve and 50.4 for TNF-IR patients. After 24 weeks of treatment in bDMARD-naive patients, the mean change from baseline in DAPSA was -25.7 (IXE Q4W), -30.0 (IXE Q2W), -22.6 (ADA) versus -10.7 (PBO; p<0.001 vs PBO for all comparisons; FIGURE). Similarly, in TNF-IR patients, mean change from baseline in DAPSA was -30.6 (IXE Q4W), -27.7 (IXE Q2W) vs -14.7 (PBO; p<0.001 vs PBO for all comparisons; FIGURE). In both populations, significant differences versus PBO were observed as early as Week 1. Furthermore, improvements persisted through 52 weeks with changes of -31.0 (IXE Q4W) and -33.8 (IXE Q2W) in the bDMARD-naive population and -31.7 (IXE Q4W) and -32.1 (IXE Q2W) in the TNF-IR population from baseline to week 52. Consistent, rapid and sustained improvements were observed with each component of the DAPSA.
Conclusion: Ixekizumab treatment resulted in significant and rapid improvements in DAPSA and its components from week 1 to week 24 which persisted through 52 weeks in 2 independent trials, SPIRIT-P1 (bDMARD-naïve patients) and SPIRIT-P2 (TNF-IR patients).
To cite this abstract in AMA style:
Sunkureddi P, Zhu B, Ogdie A, Spraberry A, Lisse J, Lin CY, Shrom D. Ixekizumab Treatment Results in Rapid and Sustained Improvements in the Disease Activity Index for Psoriatic Arthritis (DAPSA) in Patients Naïve to Biologic Dmards or with Previous Inadequate Response to TNF Inhibitors [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/ixekizumab-treatment-results-in-rapid-and-sustained-improvements-in-the-disease-activity-index-for-psoriatic-arthritis-dapsa-in-patients-naive-to-biologic-dmards-or-with-previous-inadequate-response/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-treatment-results-in-rapid-and-sustained-improvements-in-the-disease-activity-index-for-psoriatic-arthritis-dapsa-in-patients-naive-to-biologic-dmards-or-with-previous-inadequate-response/