Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous trials have used the Short Form (SF)-36 questionnaire to evaluate the impact of ankylosing spondylitis/radiographic axial spondyloarthritis (AS/r-axSpA) on health-related quality of life (HRQoL).1 Ixekizumab (IXE), is a high-affinity monoclonal antibody that selectively targets interleukin-17A and helps improve HRQoL in patients with AS/r-axSpA.2 The purpose of this analysis was to evaluate the effect of 52 weeks of treatment with IXE on self-reported HRQoL by SF-36 in patients with active AS/r-axSpA who fulfilled ASAS and modified New York criteria and were naïve to biologic therapy or had failed on/were intolerant to ≥1 but not more than 2, TNF inhibitors (TNF-IRs).
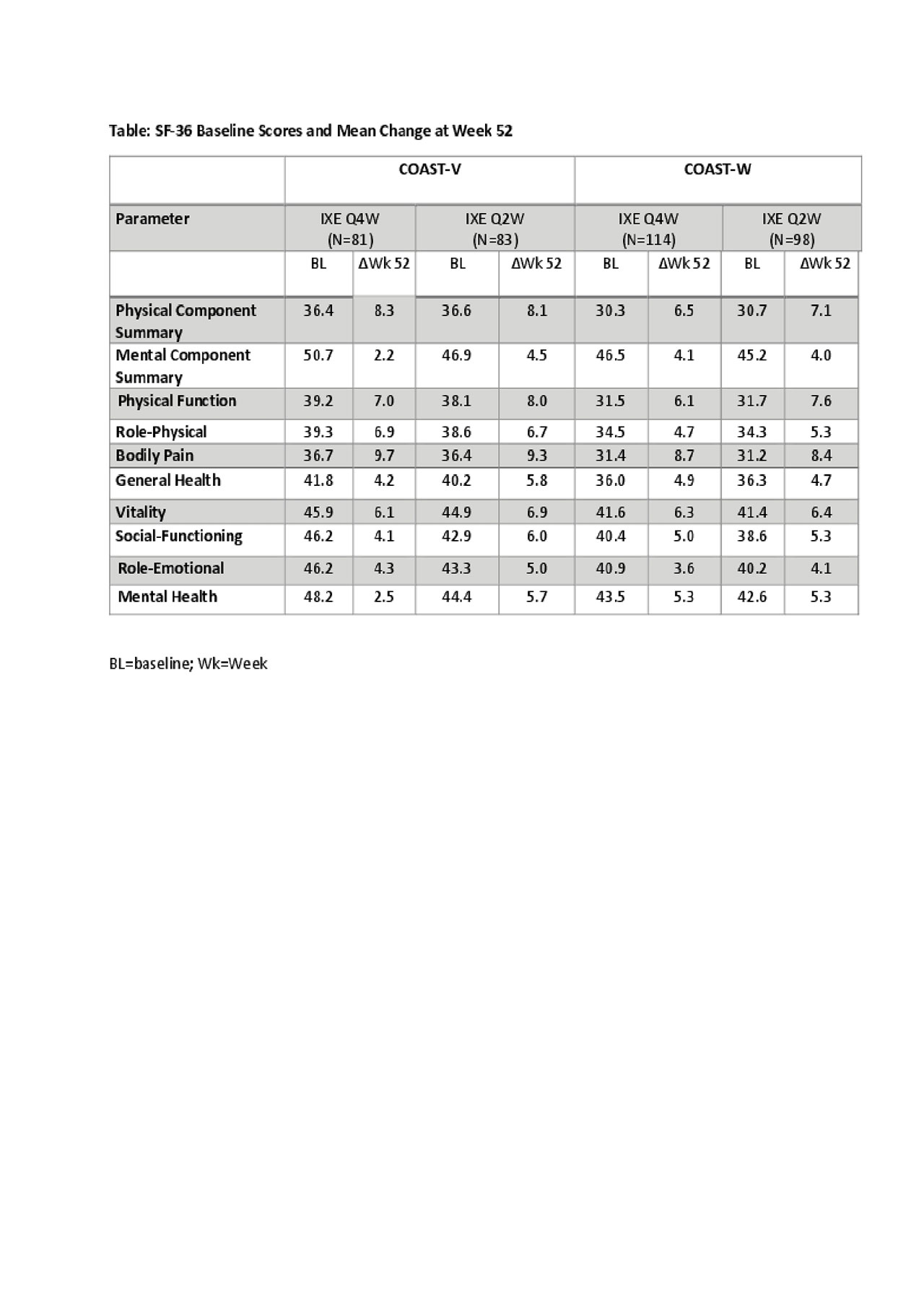
Methods: COAST-V and COAST-W are Phase 3 randomized controlled trials (RCTs) including active and placebo (PBO) comparators in the first 16 weeks in patients with active AS/r-axSpA; BASDAI and back pain scores ≥4; no prior treatment with biologic agents (COAST-V) or TNF-IR (COAST-W). During the double-blind treatment period, patients were randomized 1:1:1:1 to 80 mg IXE every 4 weeks (Q4W) or every 2 weeks (Q2W), active reference arm (adalimumab [ADA] 40mg), or PBO (COAST V) and 1:1:1 to IXE 80 mg Q4W, IXE 80 mg Q2W or PBO (COAST W). At Week 16, patients entered the extended treatment period (Weeks 16-52). Patients initially assigned to PBO/ADA were reassigned to IXEQ4W or IXEQ2W at Week 16. Patients already receiving IXE remained on their assigned treatment regimens through Week 52. Changes from baseline in SF-36 up to Week 16 were analyzed by mixed model for repeated measures. Changes from baseline in SF-36 over Weeks 36-52 were summarized using raw mean with modified baseline observation carried forward (mBOCF) for missing data imputation.
Results: Statistically significant differences in SF-36 Physical Component Summary (PCS) scores were reported in Weeks 4-16 between PBO and IXE Q4W and Q2W groups in COAST-V and COAST-W (P≤0.002 and P≤0.001, respectively). Improvements in PCS scores continued at Week 36 and sustained to Week 52 in both COAST RCTs (Figure; Table). Changes in Mental Component Summary scores were modest (Table). Improvements in all domains were reported at 52 weeks with both Q4W and Q2W doses of IXE in both trials (Table).
Conclusion: Improvements in patient-reported HRQoL in A/r-axSpA patients measured with SF-36 were observed at Weeks 4 and 16 and sustained up to 52 weeks of continuous IXE treatment.
To cite this abstract in AMA style:
Wei C, Hunter T, Van den Bosch F, Walsh J, Kiltz U, Dong Y, Sandoval D, Leon L, Strand V, Li X. Ixekizumab Significantly Improves Patient-reported Overall Health as Measured by SF-36 in Patients with Active Ankylosing Spondylitis/Radiographic Axial Spondyloarthritis: 52-Week Results of Two Phase 3 Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ixekizumab-significantly-improves-patient-reported-overall-health-as-measured-by-sf-36-in-patients-with-active-ankylosing-spondylitis-radiographic-axial-spondyloarthritis-52-week-results-of-two-phase/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-significantly-improves-patient-reported-overall-health-as-measured-by-sf-36-in-patients-with-active-ankylosing-spondylitis-radiographic-axial-spondyloarthritis-52-week-results-of-two-phase/