Session Information
Session Type: Plenary Session III
Session Time: 11:00AM-12:30PM
Background/Purpose: Ixekizumab (IXE), a high affinity IL-17A monoclonal antibody, previously showed efficacy in AS/radiographic-axSpA1,2. COAST-X (NCT02757352) is a phase 3 study that assessed efficacy and safety of IXE in patients (pts) with active nr-axSpA and objective signs of inflammation.
Methods: COAST-X was a 52-wk, randomized, double-blind, PBO-controlled study enrolling adults with an established diagnosis of axSpA who met ASAS classification (but not modified New York) criteria, had BASDAI ≥4, back pain ≥4, inflammation [sacroiliitis on MRI or elevated CRP >5 mg/L], and inadequate response or intolerance to NSAIDs. All images were centrally read. After stratification by country and screening MRI/CRP status, pts were randomized 1:1:1 to 80 mg IXE every 4 wks (Q4W), 80 mg IXE every 2 wks (Q2W), or PBO. Changes to conventional background medication (NSAIDs, csDMARDs, analgesics, and low dose corticosteroids) as well as escape to open label (OL) IXE Q2W were allowed at investigator discretion after Wk 16. Subsequent escape to OL TNF-inhibitor treatment was permitted after ≥8 wks of OL IXE Q2W. Primary endpoints were ASAS40 at Wks 16 and 52. Patients missing data or switched to OL IXE Q2W were imputed as non-responder. A logistic regression model with nonresponder imputation was used for categorical data. A mixed effects model of repeated measures was used for continuous variables. Analysis of covariance was used for sacroiliac joint (SIJ) MRI SPARCC scores.
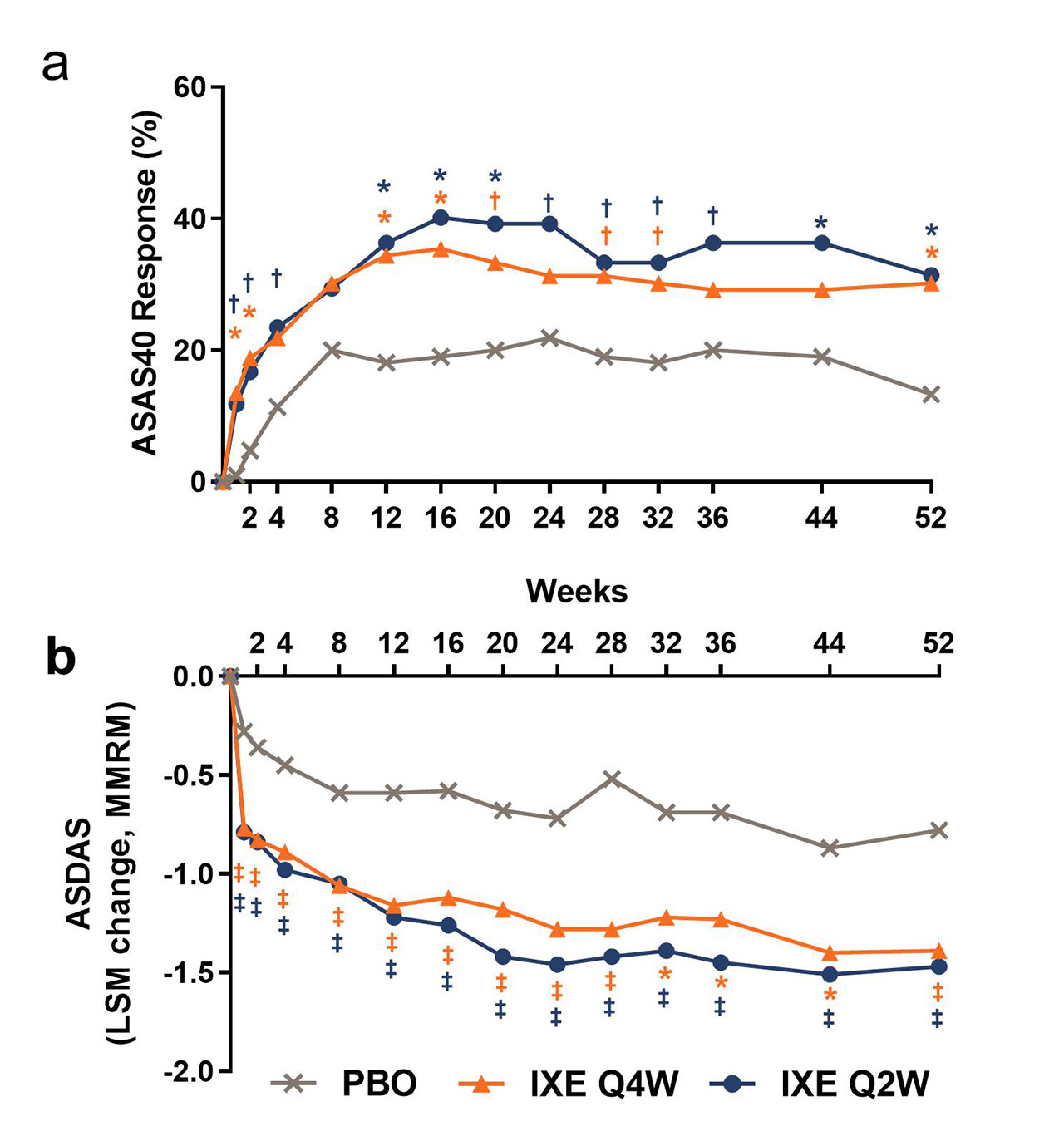
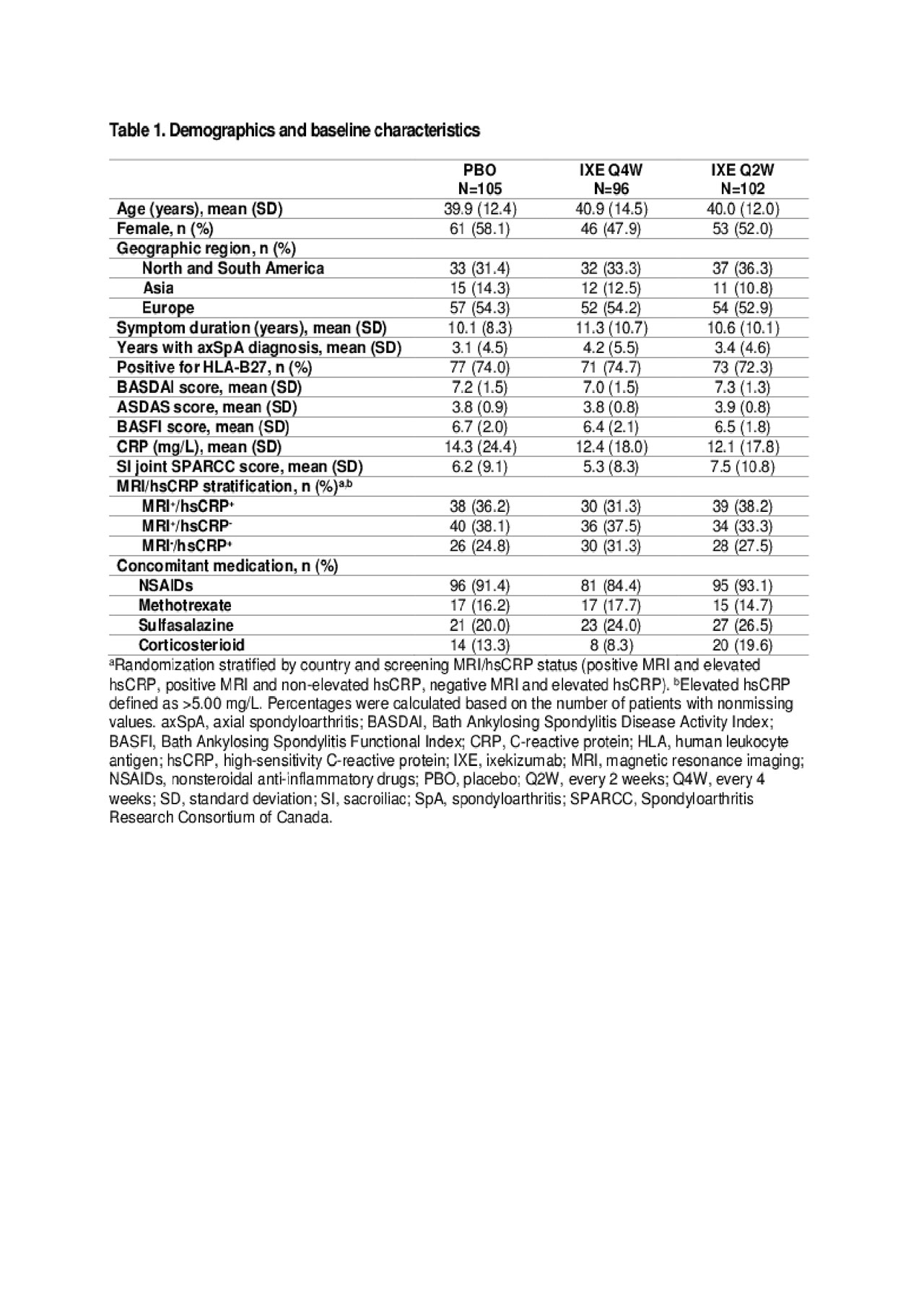
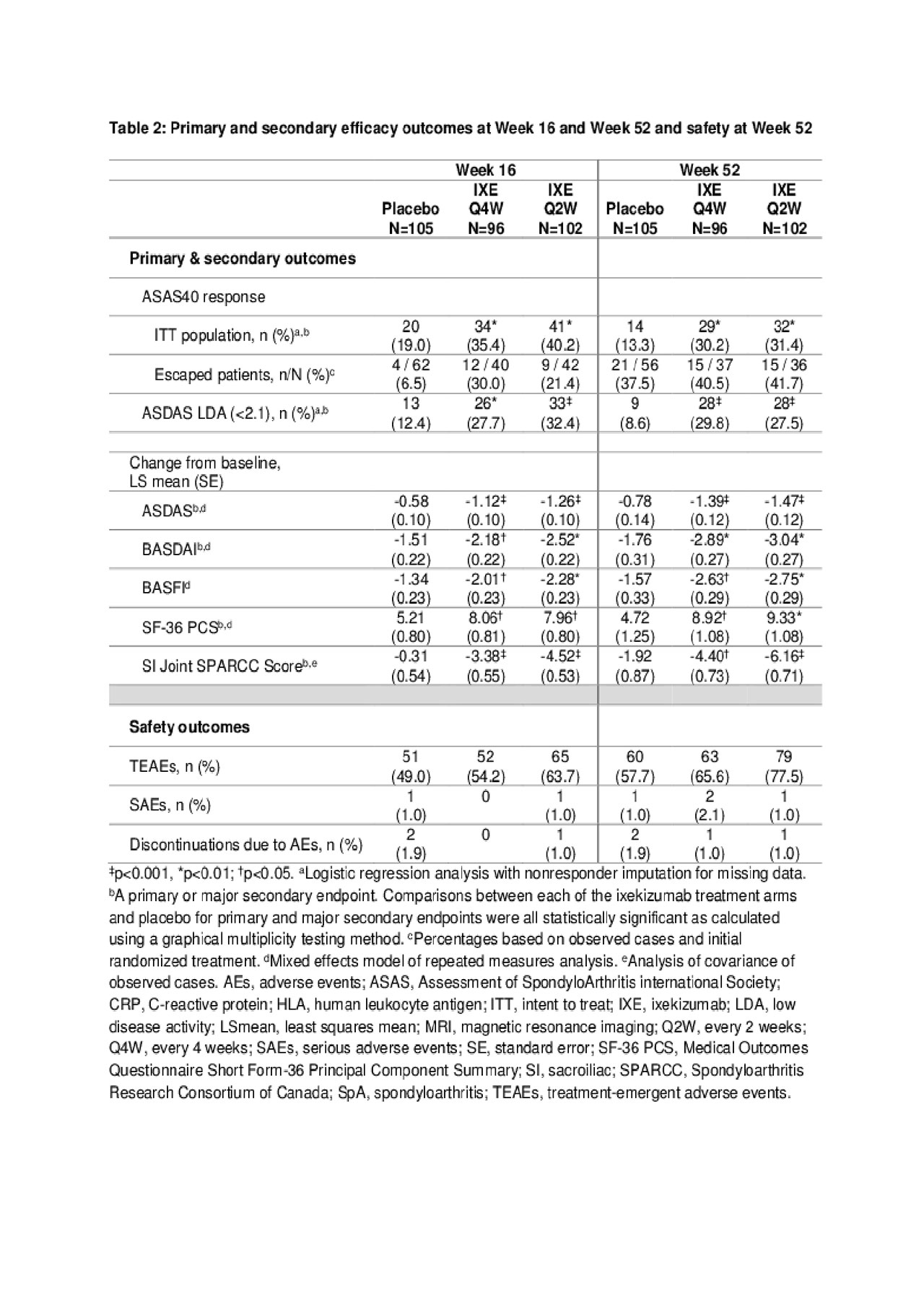
Results: Table 1 shows baseline characteristics; 303 subjects were randomized: PBO (N=105), IXE Q4W (N=96), IXE Q2W (N=102). Significantly more pts achieved ASAS40 at Wk 16: IXE Q2W (40%), IXE Q4W (35%) vs PBO (19%, p< 0.01) and at Wk 52: IXE Q2W (31%), IXE Q4W (30%) vs PBO (13%, p< 0.01) (Fig. & Table 2). Compared to PBO, pts on either IXE regimen had significantly greater changes from baseline at Wk 16 and Wk 52 for disease activity, functional status, and SIJ SPARCC scores (Fig. & Table 2). Statistically significant improvements for both IXE regimens vs PBO were first observed at Wk 1 for ASAS40. A notable proportion of pts who escaped to OL IXE Q2W had ASAS40 response at the time of escape (16.7%, 25%, and 6.5% on IXE Q2W, IXEQ4W, and PBO, respectively), and ASA40 rates increased further on open label IXE Q2 (Table 2). The frequency of serious adverse events and adverse events that led to treatment discontinuation was low and similar across all arms (Table 2). No new safety signal was identified. Conclusion: The primary endpoint of ASAS40 and all major secondary endpoints for IXE Q4W and Q2W were met at Wk 16 and Wk 52 with no unexpected safety findings. IXE added to conventional background medication was superior to conventional background medication and PBO for improving signs, symptoms, and inflammation on MRI in pts with nr-axSpA.
References
- Deodhar, et al. (2018). Arthritis Rheumatol. 71(4):599-611.
- van der Heijde, et al. (2018). Lancet. 392(10163):2441-51.
To cite this abstract in AMA style:
Deodhar A, van der Heijde D, Gensler L, Kim T, Maksymowych W, Østergaard M, Poddubnyy D, Marzo-Ortega H, Bessette L, Tomita T, Gallo G, Adams D, Leung A, Zhao F, Hojnik M, Carlier H, Sieper J. Ixekizumab in Non-Radiographic Axial Spondyloarthritis: Primary Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ixekizumab-in-non-radiographic-axial-spondyloarthritis-primary-results-from-a-phase-3-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-in-non-radiographic-axial-spondyloarthritis-primary-results-from-a-phase-3-trial/