Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinical trials have provided robust evidence of the efficacy of ixekizumab, an IL-17 inhibitor, in improving the signs and symptoms of psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) (1, 2). However, real-world evidence on ixekizumab effectiveness in spondyloarthritis is still limited (3). In 2018, ixekizumab was marked for use in PsA in Denmark/EU, and in 2020 for axSpA. In the Danish nationwide DANBIO registry, adult patients with PsA and axSpA are prospectively monitored during their disease course (4).
This study aims to explore ixekizumab treatment patterns and one-year retention rates in patients with axSpA and PsA monitored in the DANBIO registry.
Methods: Using DANBIO data, PsA and axSpA patients starting ixekizumab treatment were identified (1st of June 2017 – 13th of March 2024). Treatment patterns according to patient characteristics at treatment start (baseline) were described. Follow-up was until one year after baseline, death or last visit in DANBIO, whichever came first. We performed Kaplan-Meier estimation (log-rank tests) and adjusted Cox regression analyses (sex, age, disease duration, start year of ixekizumab, prior number of b/tsDMARDs and C-reactive protein (CRP)) to estimate the one-year ixekizumab retention rates, stratified by diagnosis (PsA, axSpA).
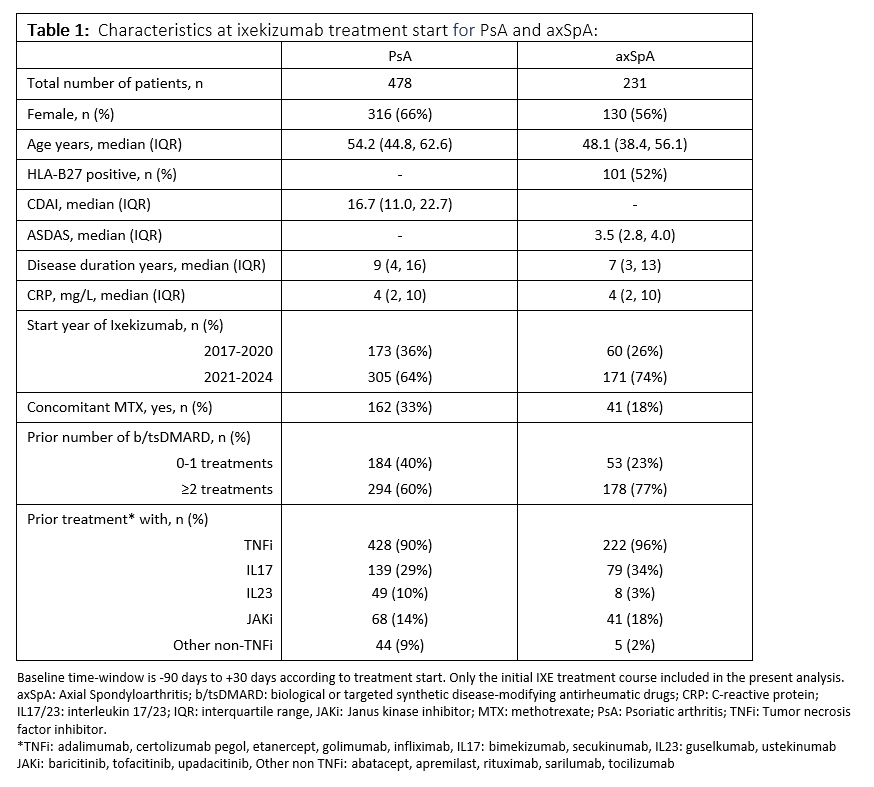
Results: At treatment start, patients with PsA had a median age of 54.2 years and a disease duration of 9.0 years, for axSpA it was 48.1 years and 6.5 years, respectively (Table 1).
For PsA and axSpA, 61% and 77% were previously treated with ≥2 b/tsDMARDs. Most patients had received TNFi prior to ixekizumab treatment (PsA: 90%; axSpA: 96%), while a third had received IL17 inhibitors (secukinumab) (PsA: 29%; axSpA: 34%) and ≤10% had received IL23 inhibitors (PsA: 29%; axSpA: 34%). For PsA, 34% of patients received concomitant methotrexate, whereas it was 18% for axSpA.
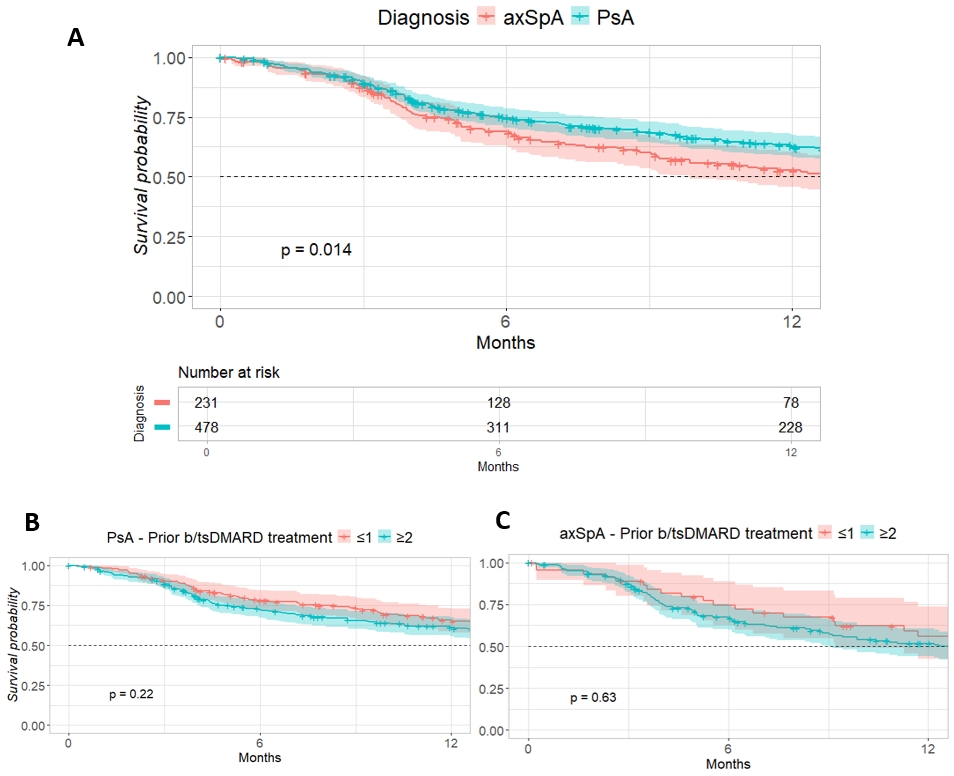
The one-year retention rate was higher for PsA than for axSpA (60.0% versus 52.6%, Figure 1). Prior number of b/tsDMARDs only had a marginal effect in both PsA and axSpA.
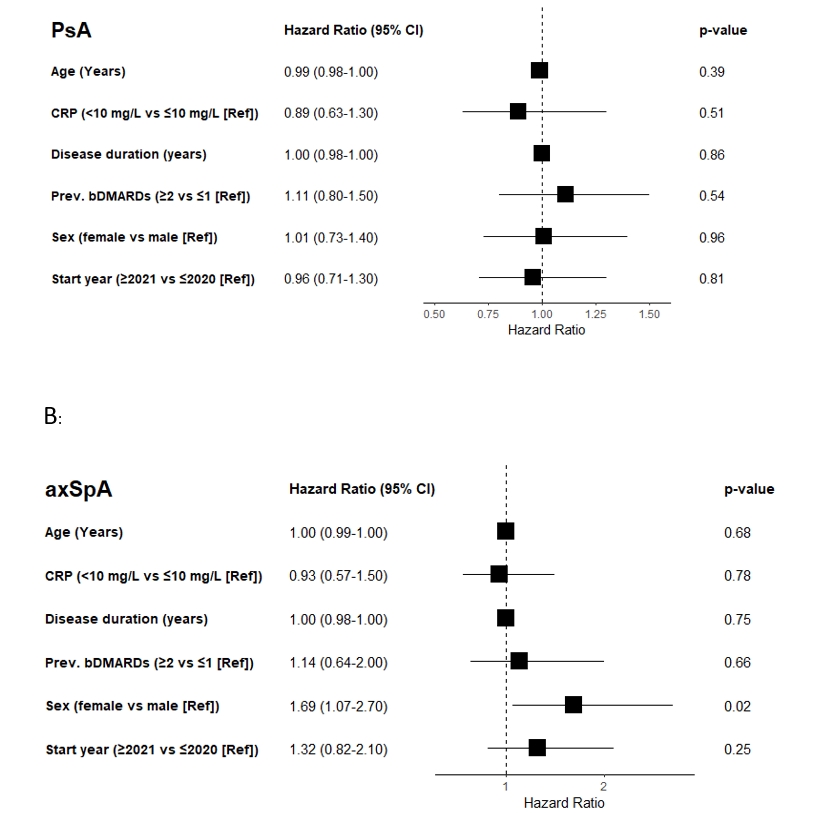
For patients with PsA, no baseline factors were associated with ixekizumab treatment discontinuation. For axSpA, female sex (adjusted hazard ratio: 1.69) was associated with discontinuation (Figure 2).
Conclusion: In this nationwide cohort study in Danish patients with PsA and axSpA, ixekizumab treatment was mainly used in patients highly experienced to previous b/tsDMARDs including TNFi and secukinumab. At one year, retention to ixekizumab treatment was higher in PsA than axSpA and was largely similar irrespective of number of previous b/tsDMARDs. For axSpA, female sex was associated with poorer retention. The results show promising results for ixekizumab treatment effects despite the failure of several previous b/tsDMARD – even within other IL17 inhibitors.
References
- Deodhar A, et al. J Rheumatol. 2023
- Deodhar A, et al. Ther Adv Musculoskelet Dis. 2023
- Egeberg A, et al. Semin Arthritis Rheum. 2022
- Ibfelt EH, et al. Clin Epidemiol. 2017
* Merete Hetland and Bente Glintborg are shared last author.
Acknowledgements
We thank all contributors to the DANBIO registry and Niels Steen Krogh, Zitelab, for the data management.
To cite this abstract in AMA style:
Jensen K, Hetland M, Glintborg B. Ixekizumab for the Treatment of Patients with Spondyloarthritis – Real-world Evidence from the Nationwide Danish Registry, DANBIO [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ixekizumab-for-the-treatment-of-patients-with-spondyloarthritis-real-world-evidence-from-the-nationwide-danish-registry-danbio/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-for-the-treatment-of-patients-with-spondyloarthritis-real-world-evidence-from-the-nationwide-danish-registry-danbio/