Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic, systemic inflammatory disease with signs and symptoms across multiple domains, including cutaneous manifestations, which can impact health-related quality of life (HRQoL). Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. In two Phase 3 randomized studies, patients (pts) with active PsA treated with tofacitinib experienced greater improvements in various dermatologic endpoints, compared with placebo. As pruritus is a bothersome symptom of skin disease in pts with PsA, we sought to determine how tofacitinib treatment affects pt-reported HRQoL via clinical improvements in dermatologic symptoms including itch.
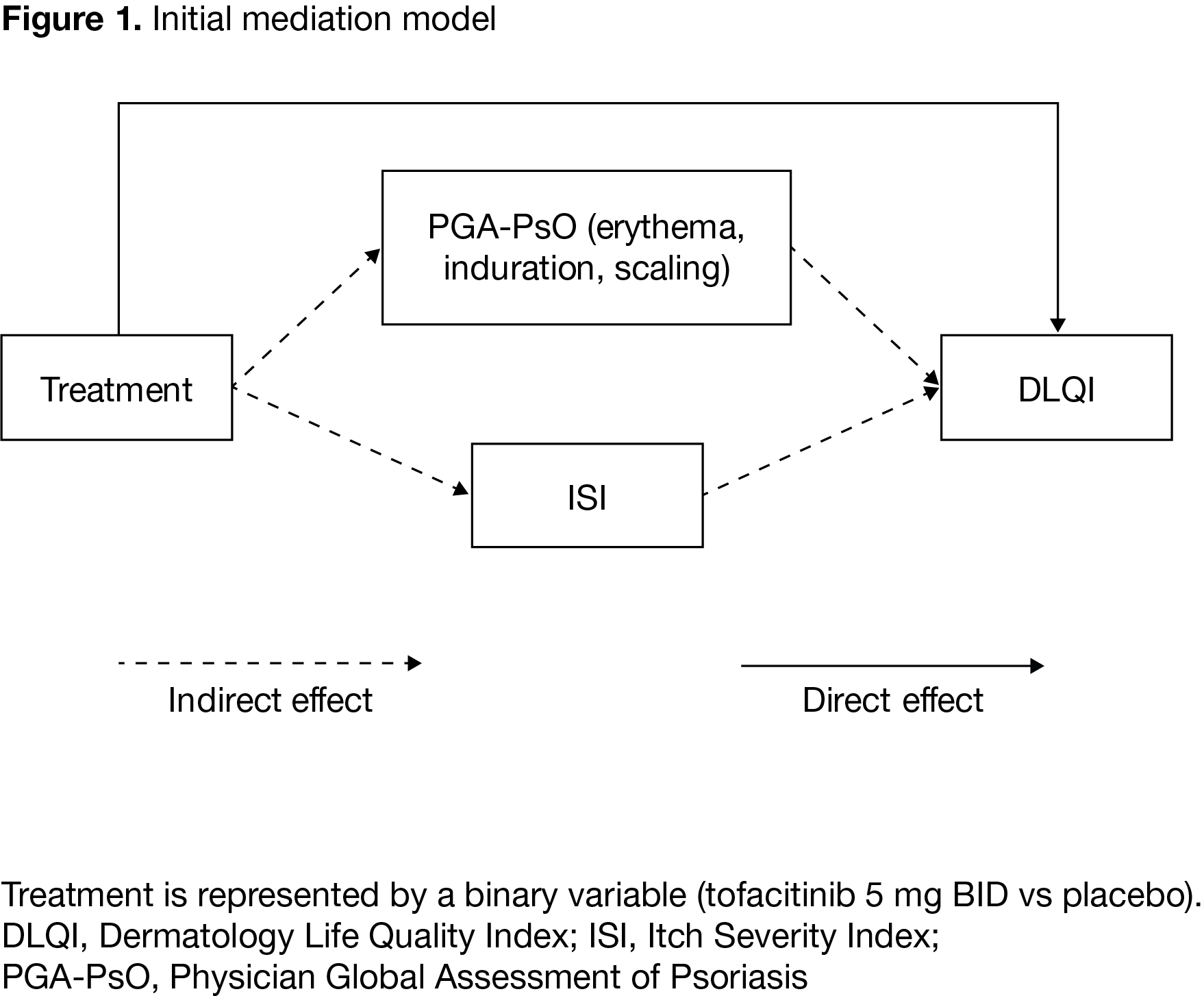
Methods: Analyses used data (mean scores from Months 1 and 3) from two Phase 3 studies (OPAL Broaden [NCT01877668]; OPAL Beyond [NCT01882439]) of pts with active PsA treated with tofacitinib 5 mg twice daily or placebo; pts were tumor necrosis factor inhibitor (TNFi)-naïve or had previous inadequate response to ≥1 TNFi. All pts were treated continuously with a single conventional synthetic DMARD. Mediation modeling, a statistical method used to assess mechanisms underlying observed relationships between different variables via other explanatory variables (mediators), was applied. The mediation model included: treatment, as the independent (explanatory) binary variable (tofacitinib 5 mg BID vs placebo); HRQoL, measured by Dermatology Life Quality Index (DLQI), as the dependent (outcome) variable; and two mediators, pt-reported Itch Severity Index (ISI) and Physician’s Global Assessment of Psoriasis (PGA-PsO) (a latent variable represented by erythema, induration, and scaling). The initial model designated the treatment effect on DLQI mediated via ISI and PGA-PsO as an indirect effect, and treatment effects not attributable to ISI or PGA-PsO as a direct effect (Figure 1).
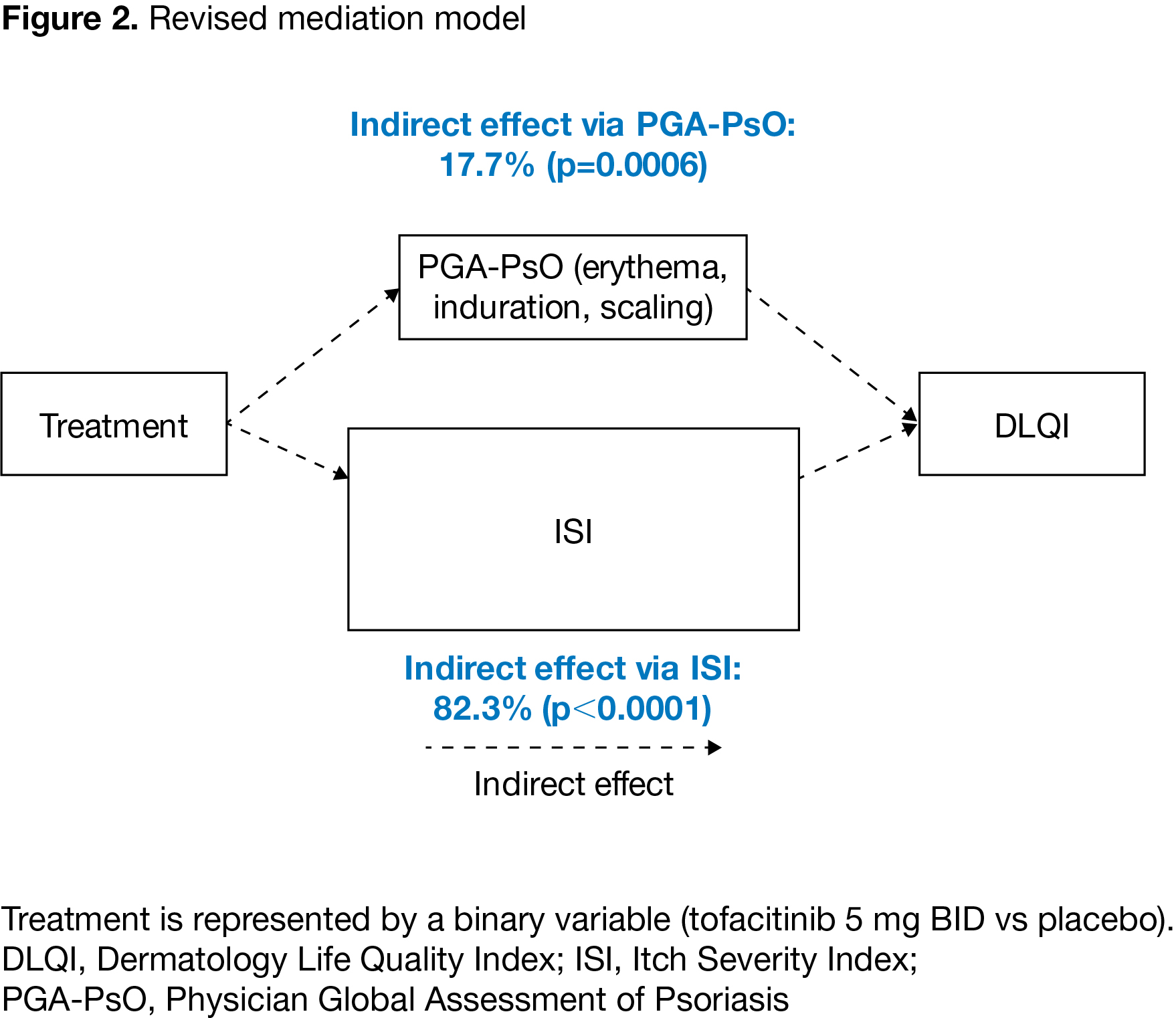
Results: Data were collected from 468 pts, pooled from both studies. In the initial model (pooled data), the effect of tofacitinib treatment on DLQI was largely mediated by itch (measured by ISI) and PGA-PsO (indirect effect) (p< 0.0001); the effect of treatment attributable to factors other than ISI and PGA-PsO (direct effect) was not statistically significant (p=0.66). Results were consistent for pooled and individual study data. Because the direct effect was small and not statistically significant, the model was re-specified to exclude the direct effect of tofacitinib treatment on DLQI. In the revised model (pooled data), 17.7% of the indirect effect of treatment on DLQI was attributable to PGA-PsO (p=0.0006) and 82.3% was attributable to itch (p< 0.0001) (Figure 2). Analyses of individual studies using the revised model gave results generally consistent with pooled data.
Conclusion: Dermatology-focused mediation modeling showed that the majority of the effect (~80%) of tofacitinib treatment on DLQI is mediated by improvements in itch, with ~20% mediated via improvements in PGA-PsO.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Eric Comeau, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Merola J, Taylor P, Bushmakin A, Cappelleri J, Young P, Germino R, Yosipovitch G. Itch as the Major Mediator of the Effect of Tofacitinib on Health-Related Quality of Life in Psoriatic Arthritis: A Mediation Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/itch-as-the-major-mediator-of-the-effect-of-tofacitinib-on-health-related-quality-of-life-in-psoriatic-arthritis-a-mediation-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/itch-as-the-major-mediator-of-the-effect-of-tofacitinib-on-health-related-quality-of-life-in-psoriatic-arthritis-a-mediation-analysis/