Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Traditionally in ELISA detection of APS antibodies, the use of serum is thought to be preferable over plasma according to international consensus bodies. The dilution effects of citrate in the plasma blood draw tube or matrix effects with fibrinogen are some of the cited concerns. However, there is scarcity of clinical data comparing reproducibility of APS antibody detection in serum versus plasma. The purpose of this study was to determine reproducibility of anti-cardiolipin (aCL), beta2-glycoprotein-1 (β2GP1), and phosphatidylserine-prothrombin (aPS-PT) antibody detection between serum and plasma in a real-world clinical setting.
Methods: Patients with clinical serum draws for IgG/IgM aCL, β2GP1, and aPS-PT antibodies were identified, and same-day, platelet-poor citrated plasma samples were obtained for repeat ELISA testing with commercially available ELISA assays. Quantitative levels were determined for each isotype without citrate volume correction and further stratified into Negative (< 15.0 GPL/MPL or U/mL), Weakly Positive (15.0-39.9), or Positive (≥40.0) reference cutoff categories. Median and mean differences along with inter-sample reliability were compared using Wilcoxon paired signed-rank method and kappa coefficients, respectively.
Results: One hundred fifty patients were identified for study with 134 aCL, 110 β2GP1, and 7 aPS-PT serum samples eligible for repeat plasma testing. Mean age was 49±16 years. About 69% were female, 87% were Caucasian, 21% with APS, and 13% with SLE. As shown in Table 1, median differences were generally 0.0 units and mean differences largely falling within 2 units. The differences were technically statistically significant. There was strong inter-sample agreement in reference cutoff category with kappa coefficients ranging from 0.80 to 1.00.
Conclusion: To our knowledge, we present the largest study examining the reproducibility of APS antibodies on paired serum and plasma samples in a real-world clinical setting. Small differences were detected between serum and plasma for aCL and β2GP1 antibodies with similar trends in aPS-PT antibodies. Although the observed differences were statistically significant, the magnitude of the differences was small and the clinical relevance is likely negligible. Moreover, with kappa coefficients of at least 0.80, there was strong inter-sample agreement in reporting negative, weakly positive and positive categories for aCL and β2GP1 antibodies. These results suggest serum and plasma could be used interchangeably for ELISA detection of antiphospholipid antibodies in a clinical setting.
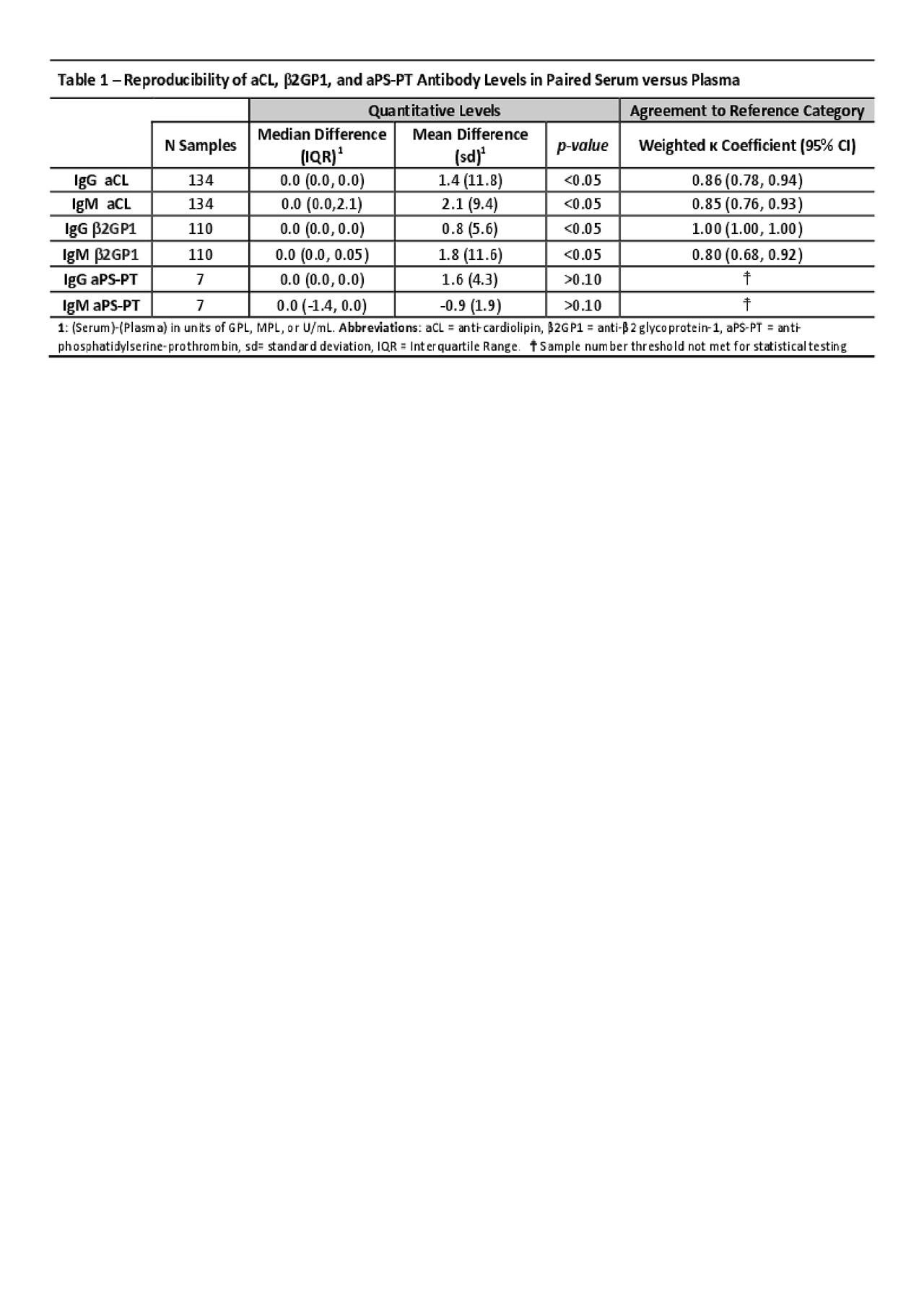
zACR_Serum vs Plasma APS antibodies_PDF TABLE
To cite this abstract in AMA style:
Pham M, Orsolini G, Crowson C, Snyder M, Pruthi R, Moder K. Is There Clinically Relevant Plasma Interference with ELISA Detection of APS Antibodies? Reproducibility of Serum and Plasma Testing in a Real-World Clinical Setting [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/is-there-clinically-relevant-plasma-interference-with-elisa-detection-of-aps-antibodies-reproducibility-of-serum-and-plasma-testing-in-a-real-world-clinical-setting/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-there-clinically-relevant-plasma-interference-with-elisa-detection-of-aps-antibodies-reproducibility-of-serum-and-plasma-testing-in-a-real-world-clinical-setting/
