Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor (TNF)-α inhibitors are extensively utilized in the treatment of inflammatory rheumatic diseases. These agents increase the risk of tuberculosis (TB), especially in countries with a high incidence, such as Turkey. This study aims to evaluate the incidence of tuberculosis after anti-TNF-α treatment for Behcet’s disease (BD) and to report the risk in comparison to other disorders in our center.
Methods: The data of 1302 patients who received anti-TNF-α treatment for more than six months between 2005 and 2018 were assessed retrospectively. Demographic features, type of aTNF-α treatment used, treatment duration, tuberculin skin test (TST) and Quantiferon results, use of isoniazid prophylaxis and the development of tuberculosis were recorded. We compared the disease characteristics of patients with and without tuberculosis after aTNF-α treatment.
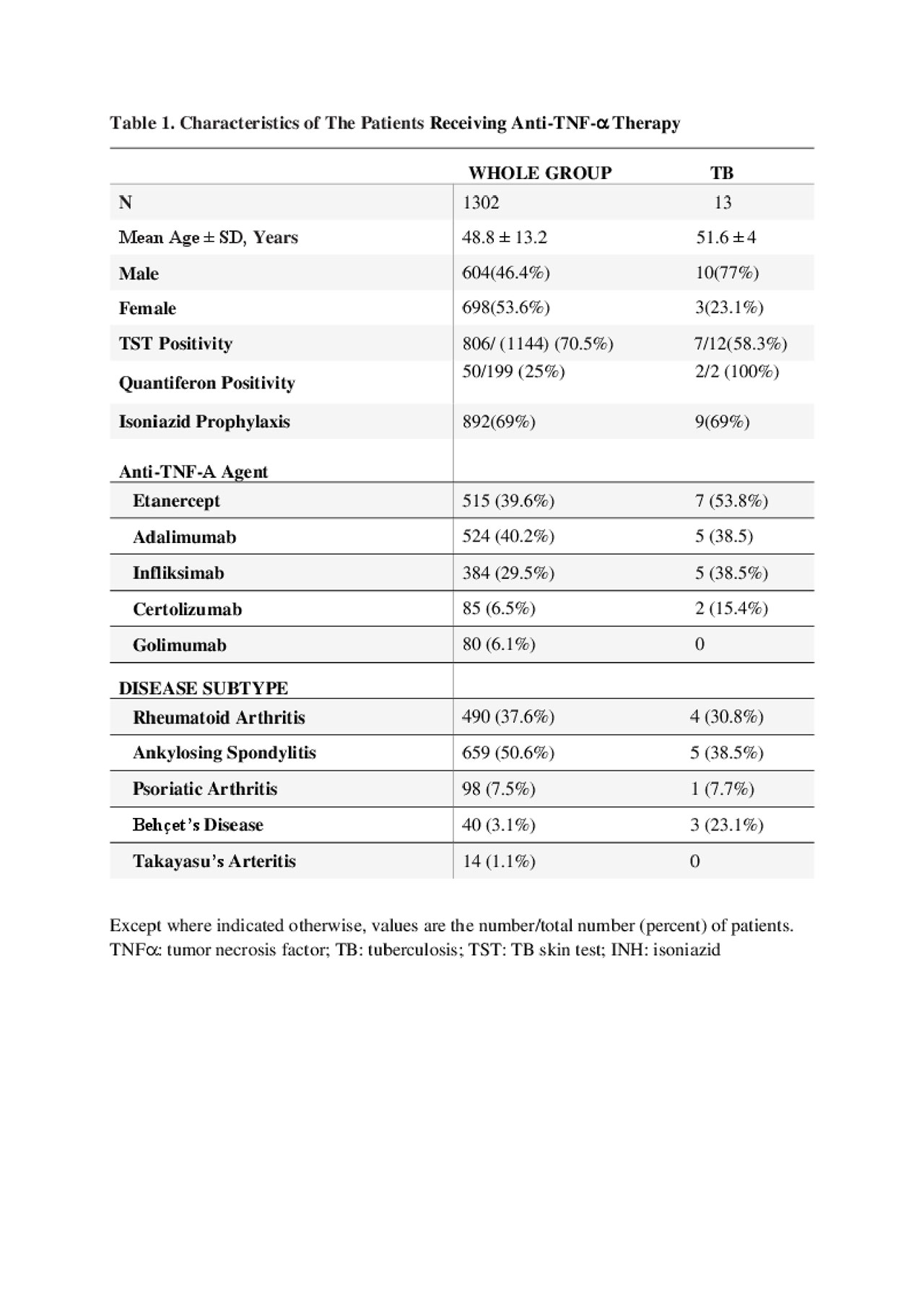
Results: Among the 1302 patients (F/M: 698/604, mean age: 48.8±13.2 years), aTNF-α agents were most commonly used in ankylosing spondylitis (AS) patients (n=659, 50.9%). Median aTNF-α treatment duration in all patients was 32.5 months (Q1-Q3: 12-61). During the follow-up period, 13 patients developed tuberculosis. Seven (54%) of the TB patients had pulmonary and six (46%) had extrapulmonary TB. Median time from the initiation of aTNFα therapy to the diagnosis of TB was 40 months (Q1-Q3: 22-56). The characteristics of all patients receiving aTNFα therapy and developed TB were displayed in Table 1. The TNFα antagonists patients receiving were Etanercept in 4, Adalimumab in 5, Infliximab in 2 and Certolizumab in 2 patients.
TB was detected in 3 (7.5%) of 40 BD patients and 10 (0.8%) of 1256 non-Behçet’s patients. There was a statistically significant increase in TB risk, in Behcet’s patients after aTNF-α treatment when compared to the rest of the group (p=0.006). When we combined our patients with a large, multicenter cohort from Turkey, among 8320 patients who received aTNF-α treatment(1), TB developed in 8 (4.9%) patients among 161 BD and 75 (0.9%) patients in 8159 non-Behçet’s patients (p=0.0002).
Conclusion: Comparable to the previous literature from Turkey, our results verified an increased risk of tuberculosis after aTNF-α treatment in patients with Behçet’s Disease compared to other rheumatological disorders. Pro-inflammatory nature of BD with a higher risk for infections or concomitant therapies such as corticosteroids might influence the higher TB risk in BD. This observation should be taken into account when anti-TNF-a agents are used in refractory BD patients with major organ involvement.
(1) Kisacik B, et al. Characteristics Predicting Tuberculosis Risk under Tumor Necrosis Factor-α Inhibitors: Report from a Large Multicenter Cohort with High Background Prevalence. J Rheumatol. 2016 Mar;43(3):524-9
To cite this abstract in AMA style:
Gazel U, Kocakaya D, Topcu I, Karatas H, Karabacak M, Atagunduz P, Inanc N, Alibaz-Oner F, Direskeneli H. Is the Risk of Tuberculosis Increased in Behçet’s Disease Compared to Other Rheumatological Disorders After Anti-TNF-α Treatment? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/is-the-risk-of-tuberculosis-increased-in-behcets-disease-compared-to-other-rheumatological-disorders-after-anti-tnf-%ce%b1-treatment/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-the-risk-of-tuberculosis-increased-in-behcets-disease-compared-to-other-rheumatological-disorders-after-anti-tnf-%ce%b1-treatment/