Session Information
Date: Monday, November 6, 2017
Title: Epidemiology and Public Health Poster II: Rheumatic Diseases Other than Rheumatoid Arthritis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Knee OA patients may undergo intra-articular (IA) hyaluronic acid (HA) injection treatment, but there is debate about its effectiveness. We asked: (1) What is the epidemiology of IA HA use in knee arthroplasty (KA) patients? (2) Is hylan G-F 20 associated with a delay to KA? (3) Is there a difference in the delay to KA with the number of HA courses?
Methods: A retrospective, observational dataset (Optum Clinformatics; 2006 to June 2016) was used to identify knee OA patients. Three different cohorts were identified: (1) No HA cohort who did not receive any IA HA; (2) Non-hylan G-F 20 cohort who received multiple HA types or only one type of non-hylan G-F 20 HA; and (3) hylan G-F 20 cohort who received only hylan G-F 20. Patients who subsequently received a KA were further identified. A quantile regression model was used, adjusting for clinical confounding factors and with propensity score weighting, to evaluate the effect of the covariates on the median duration from knee OA to KA. The trend in time to KA with each additional course of HA was also evaluated.
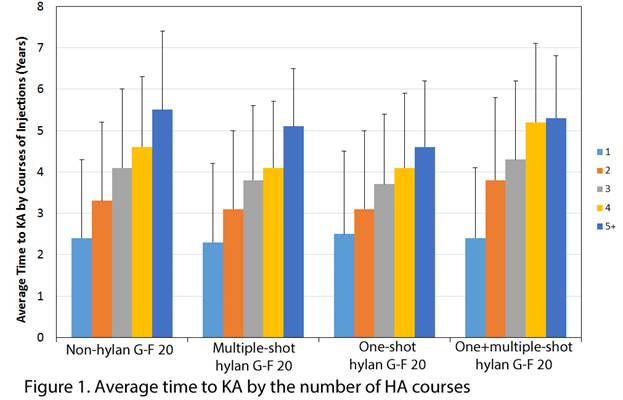
Results: From the initial cohort of 4,027,848 knee OA patients, 141,305 KA patients were identified. Overall median time from knee OA diagnosis to KA was 1.2 years (average: 1.9 ± 1.9 years) with a corresponding interquartile range of 0.4 to 2.8 years. After propensity score adjustment, HA patients had significantly longer median time to KA by at least 5 months (p<0.001; Table 1). When the HA cohort was compared after adjusting for the time to HA and number of injections, no significant differences in the time to KA between multiple-shot hylan G-F 20 and non-hylan G-F 20 HA patients were observed (p≥0.620), but a longer time was observed for one-shot hylan G-F 20 patients by about 3 months and patients who received both one- and multiple- shot hylan G-F 20 (“One+multiple-shot hylan G-F 20”) by about 15 to 16 months. There was a trend toward longer time to KA the more HA courses the patient underwent (Figure 1). For example, the average time to KA increased from 2.3 years to 5.1 years as the hylan G-F 20 patients increased their treatment courses from one to five or more. There was disparity between states in terms of KA patients who used HA. The three states with the lowest percent of KA patients who used HA were HI (14.5%), IN (16.3%), and AK (16.7%), while the three states with the highest percent of KA patients who used HA were NJ (39.1%), LA (33.9%), and MS (31.8%).
Conclusion: Most KA patients did not use HA (73.7%) and the ones who received it were associated with a longer median time to KA by 5 to 22 months, depending on the HA cohort. The time to KA was shown to increase with more HA courses.
To cite this abstract in AMA style:
Ong K, Runa M, Lau E, Altman R. Is Intra-Articular Injection of Synvisc Associated with a Delay to Knee Arthroplasty in Knee OA Patients? [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/is-intra-articular-injection-of-synvisc-associated-with-a-delay-to-knee-arthroplasty-in-knee-oa-patients/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-intra-articular-injection-of-synvisc-associated-with-a-delay-to-knee-arthroplasty-in-knee-oa-patients/