Session Information
Date: Tuesday, November 14, 2023
Title: (2019–2038) Patient Outcomes, Preferences, & Attitudes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients bring valuable insights to research; the inclusion of patient research partners (PRPs) in research projects is increasingly recognised and recommended in medical research, also by the Food and Drug Administration. The level of involvement of PRPs in translational rheumatology research projects remains unknown, while in randomized clinical trials (RCTs) published between 2016 and 2020, it has been reported to be as low as 2%1. The objective of this study was to assess the involvement of PRPs in recent translational studies and RCTs in rheumatology.
Methods: In this scoping review, we analyzed the 80 most recent articles (40 for translational studies and 40 for RCTs) in each of the target diseases (rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus and lower-extremity osteoarthritis), published up until March 1st, 2023, in rheumatology and general scientific journals with an impact factor of >5. The extent of PRP involvement was assessed, as reported in the Methods, affiliations of authors and acknowledgments sections. General data on the studies was also collected. The analysis was descriptive.
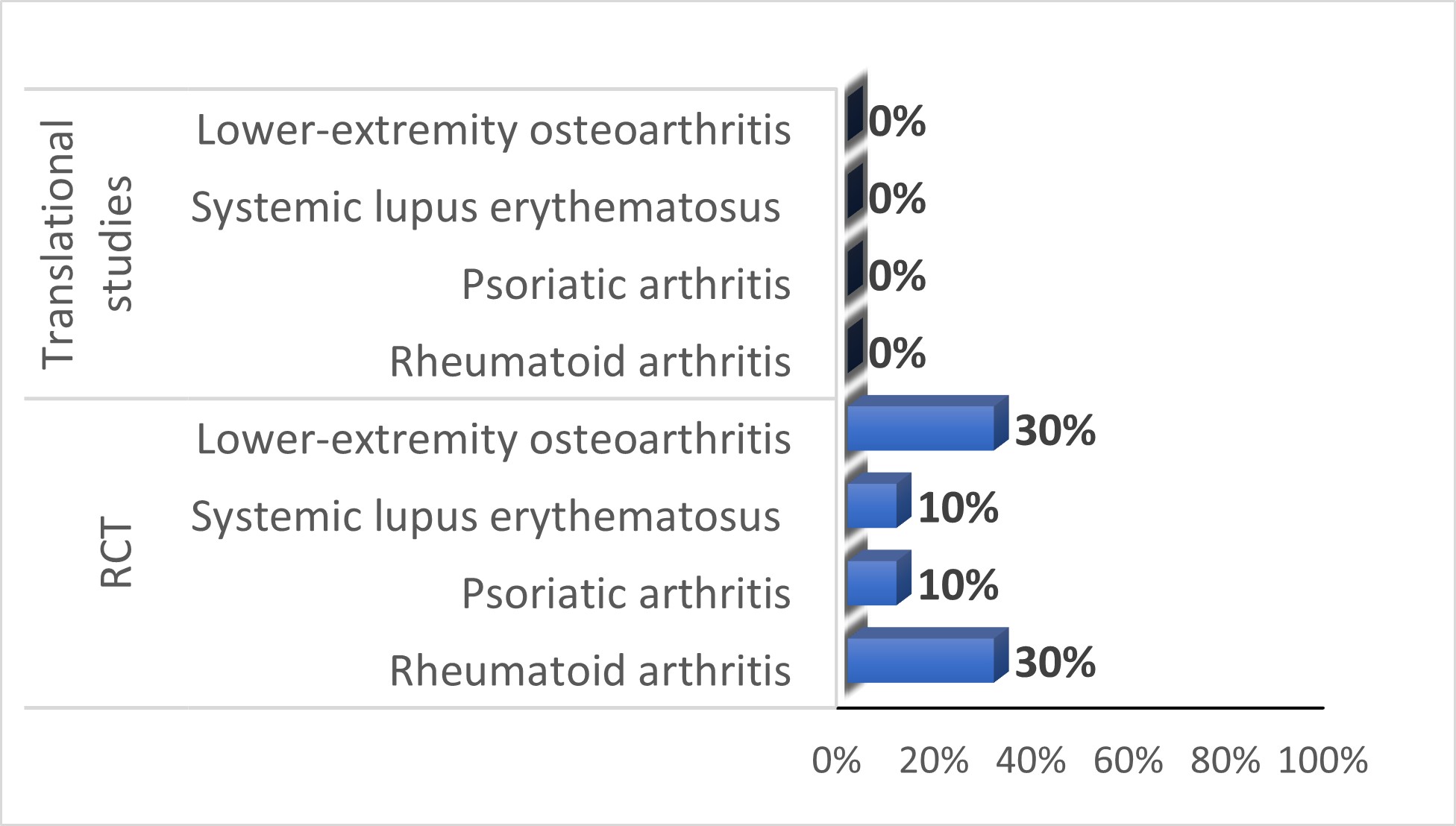
Results: Overall, 221 studies were screened; most were excluded due to wrong study design or wrong disease. Among the 40 translational studies (10 for each disease studied), half were published in rheumatological journals. Fifty percent of the studies were conducted in Asia, 30% in Europe and 20% in North America. None of the included translational studies reported PRP involvement (Figure 1). Of 40 clinical trials (10 for each disease studied), 78% were published in rheumatology journals. Fifty-two percent of the studies were conducted in North America, 25% in Europe and 23% in Asia. Among the 40 RCTs, 8 studies (20%) reported PRP involvement (Figure 1). These trials were from Europe (6/8, 75%), and North America (2/8, 25%). Most of them (6/8, 75%) were non-industry funded. The type of PRP involvement was reported in 4/8 studies (50%); four studies reported PRP participation in the study design and two studies reported involvement in the interpretation of the results. All the trials reporting the number of PRPs (6/8, 75%), involved at least two PRPs.
Conclusion: Despite a world-wide movement advocating for increased patient involvement in research, the involvement of PRPs in translational research and RCTs in rheumatology remains low. While there has been an improvement in PRP involvement in RCTs compared to previous literature reviews (20% vs. 2%)1, PRPs are still absent in translational research projects. This under-representation of PRP involvement in research highlights a persistent gap between recommendations and practice.
References:
1. Wang H, et al. Patient research partner involvement in rheumatology clinical trials: analysis of journal articles 2016–2020. Ann Rheum Dis 2021;80:1095-1096
To cite this abstract in AMA style:
Benavent D, Elhai M, Aouad K, Studenic P, de Wit M, Gossec L. Involving Patients Research Partners in Research in Rheumatology: Where Do We Stand? A Scoping Review of Recent Randomized Controlled Trials and Translational Science Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/involving-patients-research-partners-in-research-in-rheumatology-where-do-we-stand-a-scoping-review-of-recent-randomized-controlled-trials-and-translational-science-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/involving-patients-research-partners-in-research-in-rheumatology-where-do-we-stand-a-scoping-review-of-recent-randomized-controlled-trials-and-translational-science-studies/