Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Tocilizumab (TCZ) has shown efficacy in large-vessel vasculitis, including Giant Cell Arteritis (GCA). Clinical trials with TCZ in GCA were performed with intravenous (iv) TCZ in a phase 2 trial, and with subcutaneous (sc) TCZ in the phase 3 GiACTA. However, in GCA there are no studies comparing IV vs SC TCZ.
Our aim was to compare the efficacy of TCZ in GCA patients according to the route of administration IV-TCZ vs SC-TCZ.
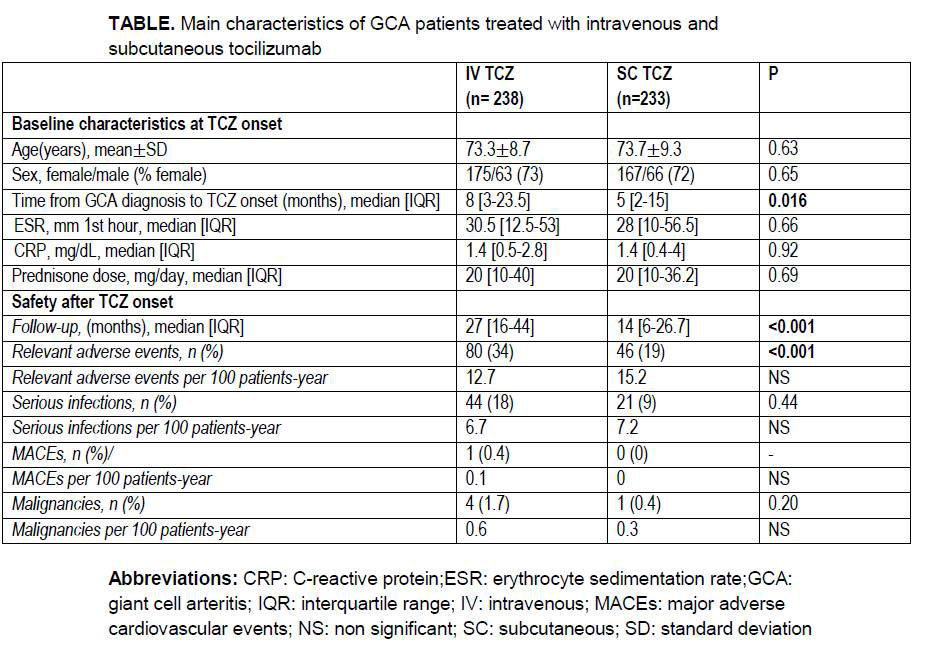
Methods: Multicentre study of 471 patients diagnosed with GCA and treated with TCZ. They were divided into 2 groups according to the route of administration: a) IV, and b) SC. GCA was diagnosed by: a) ACR criteria, and/or b) temporal artery biopsy, and/or c) imaging techniques. Sustained remission was established according to EULAR definitions.
Results: We studied 471 patients (mean age, 74 ±9 years) treated with TCZ, 238 with IV-TCZ and 233 with SC-TCZ (TABLE). The time between diagnosis of GCA and TCZ onset was shorter in the SC TCZ group. Regarding acute phase reactants at the beginning of TCZ, no differences were found between both groups. There were no significant differences in sustained remission or in glucocorticoid-sparing effect of TCZ (FIGURE). Patients on IV TCZ treatment suffered more relevant adverse effects during follow-up.
Conclusion: In GCA, TCZ seems equally effective and safe regardless of the route of administration IV or SC.
To cite this abstract in AMA style:
Sánchez-Bilbao L, Loricera J, Castañeda S, Moriano C, Narváez F, Aldasoro V, Maiz O, Melero R, Villa J, Vela-Casampere P, Romero Yuste S, Callejas J, De Miguel E, Galíndez-Agirregoikoa E, Sivera F, Fernández-López J, Galisteo C, Ferraz Amaro I, Sánchez-Martín J, Calderón-Goercke M, Hernández J, González-Gay M, Blanco R. Intravenous versus Subcutaneous Tocilizumab in a Series of 471 Patients with Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/intravenous-versus-subcutaneous-tocilizumab-in-a-series-of-471-patients-with-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intravenous-versus-subcutaneous-tocilizumab-in-a-series-of-471-patients-with-giant-cell-arteritis/