Session Information
Date: Monday, November 6, 2017
Title: Osteoarthritis – Clinical Aspects Poster I: Clinical Trials and Interventions
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Methods: This was a retrospective analysis of the Blue Cross/ Blue Shield (BCBS) claims database from October 1st, 2010 through September 30th, 2015. The primary outcome was time to TKA, which was defined as the time from treatment to the time TKA. Kaplan-Meier survival analysis was conducted to determine the TKA-free survival of patients who received IA-HA injections stratified by the number of injection courses received versus those who did not receive any IA-HA injections. A Cox proportional hazards regression analysis was also conducted to compare hazards ratios between the non IA-HA treated group and the IA-HA injection subgroups.
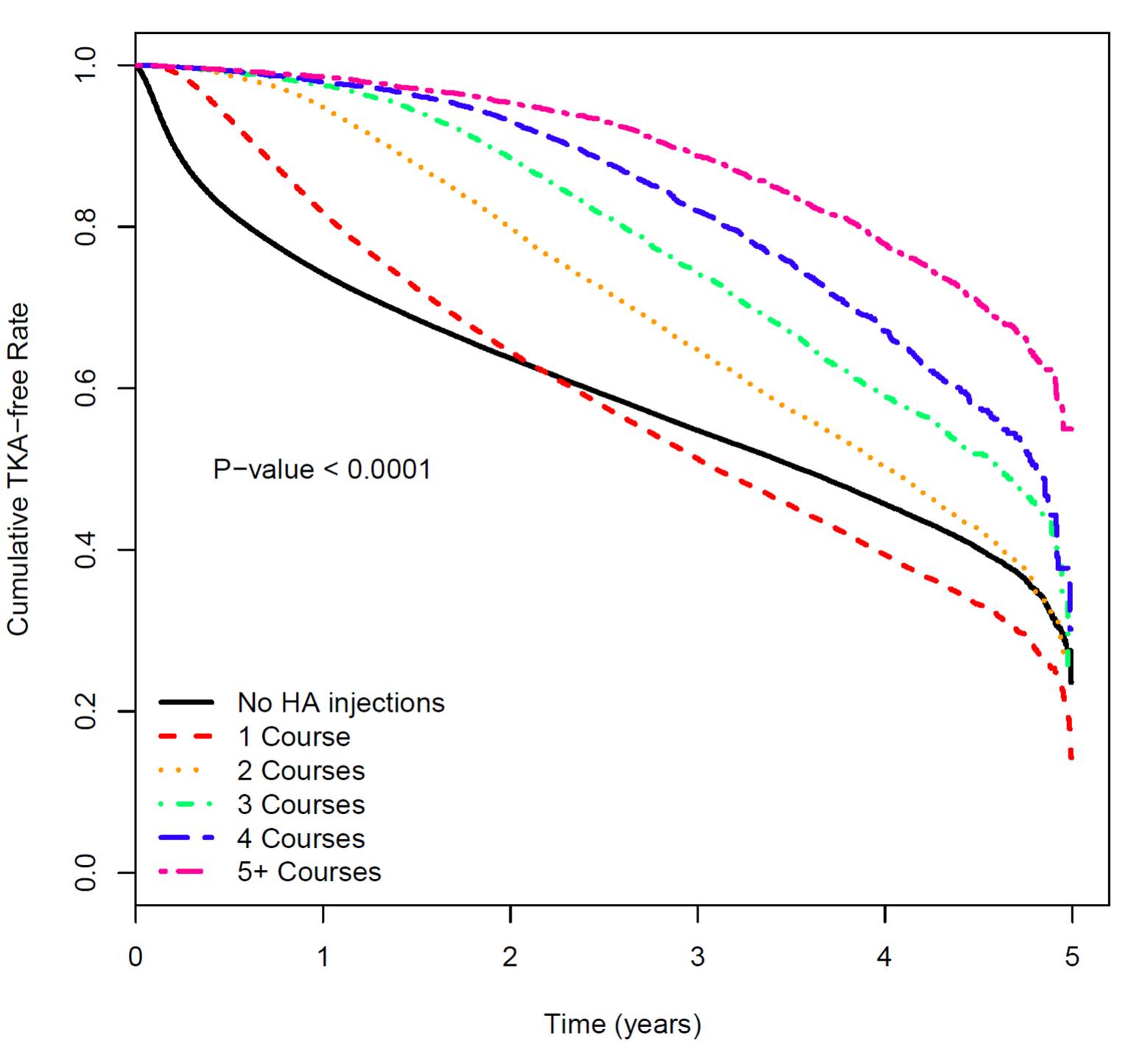
Results: A total of 744,734 patients were included in the analysis. Of these, 181,631 received at least one IA-HA injection, while 563,103 did not receive an IA-HA injection. A delay to TKA compared to the non IA-HA treated group was generally observed within the first year after IA-HA treatment for patients treated with 1 injection course, while patients treated with multiple courses demonstrated an incremental increase in delay to TKA with more injections (Figure 1). The model demonstrated a gradual decrease in hazard ratio (HR) with each subsequent course of IA-HA injection in comparison to the no IA-HA injection group, suggesting that the risk of TKA is reduced with subsequent IA-HA courses. The hazard ratio for a single course of injections was 0.85 (95% CI 0.84 – 0.86). The HR for TKA was smallest in the 5 or more injection courses group (HR 0.27, 95% CI 0.25 to 0.28).
Conclusion: These results demonstrate that within a large cohort of knee OA patients, individuals who received multiple courses of HA injections had a progressively greater delay to TKA compared to patients who did not receive HA treatment. The results demonstrated that with a greater number of injection courses received over time, there may be a relationship to a longer delay in TKA. Based on these results, multiple, repeat courses of IA-HA injection may be beneficial in substantially delaying TKA in knee OA patients.
Figure 1: Survival Analysis
To cite this abstract in AMA style:
Concoff A, Niazi F, Shaw P, Rosen J. Intra-Articular Hyaluronic Acid Delay to Total Knee Arthroplasty By Number of Injection Courses Received: Analysis of an Administrative Database [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/intra-articular-hyaluronic-acid-delay-to-total-knee-arthroplasty-by-number-of-injection-courses-received-analysis-of-an-administrative-database/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intra-articular-hyaluronic-acid-delay-to-total-knee-arthroplasty-by-number-of-injection-courses-received-analysis-of-an-administrative-database/