Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The use of intra-articular (IA) corticosteroid injections has been widely accepted as a therapy to control local inflammation. Studies on the use of IA TNF inhibitors revealed conflicting results. Case-reports suggested long-term efficacy of single or repeated injections in inflammatory arthritides. Randomized controlled trials comparing the use of IA TNF inhibitors to IA corticosteroids, showed either inferior or comparable efficacy. It is unknown if IA TNF blockade is more effective than placebo treatment. The objective of this trial was to investigate efficacy and safety of a single IA etanercept injection in comparison to placebo.
Methods: Patients with rheumatoid arthritis (RA) (revised 1987 ACR criteria) or psoriatic arthritis (PsA) (CASPAR classification criteria) and arthritis of knee, ankle, wrist, elbow or MPC joint despite a stable dose of methotrexate and/or prednisone were included. Patients were randomized to receive an IA injection of etanercept (25 mg) or placebo (0.9% NaCl) in a 2:1 ratio. Target joint improvement was determined by a composite change index (CCI; score 0-10), including a visual analogue scale (VAS) for pain, clinical assessments (tenderness, swelling and disability, scored 0-3) and patient’s and doctor’s assessment of treatment effect. To evaluate the effect of treatment on the CCI as a dependent variable, generalised estimating equations was used with age, gender, disease duration, CRP at baseline and TJC at baseline as covariates, and an exchangeable working correlation structure was chosen. Safety and general disease activity parameters were evaluated weekly up to week 6, as well as systemic CRP level, ESR and etanercept levels.
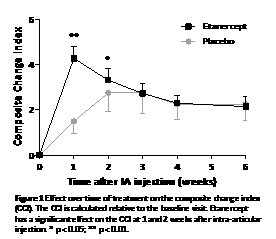 Results: Thirty-two patients (RA=12; PsA=20) were included, 22 received etanercept and 9 placebo. At baseline both groups were comparable. Treatment with etanercept resulted in a prompt and statistically significant improvement of the CCI (p<0.001) in comparison with placebo, as well as a decrease in general parameters. When analysed over time, this beneficial effect was transient and only statistically significant at week 1 and 2 after IA injection (Figure 1). Joint tenderness remained decreased up to week 6 (p < 0.01). Maximum serum etanercept levels measured after 1 week were 1.39 ± 0.46 ug/ml. Levels were comparable between CCI ‘good'- and ‘non-responders'. Mild transient adverse events were reported for 7 (32%) patients treated with etanercept and 2 (25%) with placebo (p=0,55). No serious adverse events were reported.
Results: Thirty-two patients (RA=12; PsA=20) were included, 22 received etanercept and 9 placebo. At baseline both groups were comparable. Treatment with etanercept resulted in a prompt and statistically significant improvement of the CCI (p<0.001) in comparison with placebo, as well as a decrease in general parameters. When analysed over time, this beneficial effect was transient and only statistically significant at week 1 and 2 after IA injection (Figure 1). Joint tenderness remained decreased up to week 6 (p < 0.01). Maximum serum etanercept levels measured after 1 week were 1.39 ± 0.46 ug/ml. Levels were comparable between CCI ‘good'- and ‘non-responders'. Mild transient adverse events were reported for 7 (32%) patients treated with etanercept and 2 (25%) with placebo (p=0,55). No serious adverse events were reported.
Conclusion: A single IA etanercept injection is feasible and safe and results in transient improvement of disease activity in the target joint as well as general parameters. This treatment can benefit patients who do not respond to or do not tolerate IA corticosteroids. Due to the high costs associated with anti-TNF treatment, IA corticosteroids remain the preferred treatment option.
Disclosure:
C. J. Aalbers,
None;
D. M. Gerlag,
None;
K. Vos,
None;
G. Wolbink,
Pfizer Inc,
2,
Pfizer Inc,
8,
Amgen,
8;
R. Landewe,
Abbott Immunology Pharmaceuticals, Amgen, Centocor, BMS, Johnson-Johnson, Merck, Pfizer, Roche,
5;
P. P. Tak,
Employee of GlaxoSmithKline,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intra-articular-etanercept-treatment-in-inflammatory-arthritis-a-randomized-double-blind-placebo-controlled-trial/
