Session Information
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Rheumatoid arthritis (RA) is the most common autoimmune disease and requires long term treatment to suppress inflammation. Currently, treatment is started when arthritis is clinically apparent. We hypothesized that earlier intervention, in the preceding phase of arthralgia and subclinical joint inflammation, prevents the development of clinical arthritis and reduces the disease burden.
Methods: In this randomised, double blind, two-year, proof-of-concept trial, adults with arthralgia clinically suspected of progressing to RA and MRI-detected subclinical joint inflammation, recruited from all rheumatology outpatient clinics in the southwest-Netherlands, were assigned (1:1) to a single intramuscular glucocorticoid-injection (120 mg) and a one-year course of oral methotrexate (up-to 25mg/week), or placebo (injection and tablets). Subsequently, participants were followed for another year. Primary endpoint was the development of clinical arthritis (fulfilling the 2010-RA-criteria or involving ≥2 joints) that persisted ≥2 weeks. Patient-reported physical functioning, symptoms and work productivity, were key secondary endpoints and measured 4-monthly. Additionally, the course of MRI-detected inflammation was studied. Two prespecified subgroup analyses were performed: analyses of participants with high-risk of developing clinical arthritis (PPV ≥70%), and analyses stratified for ACPA-status. All participants entered the intention-to-treat-analysis. The trial is registered with the Netherlands Trials Registry (NTR4853 trial NL4599).
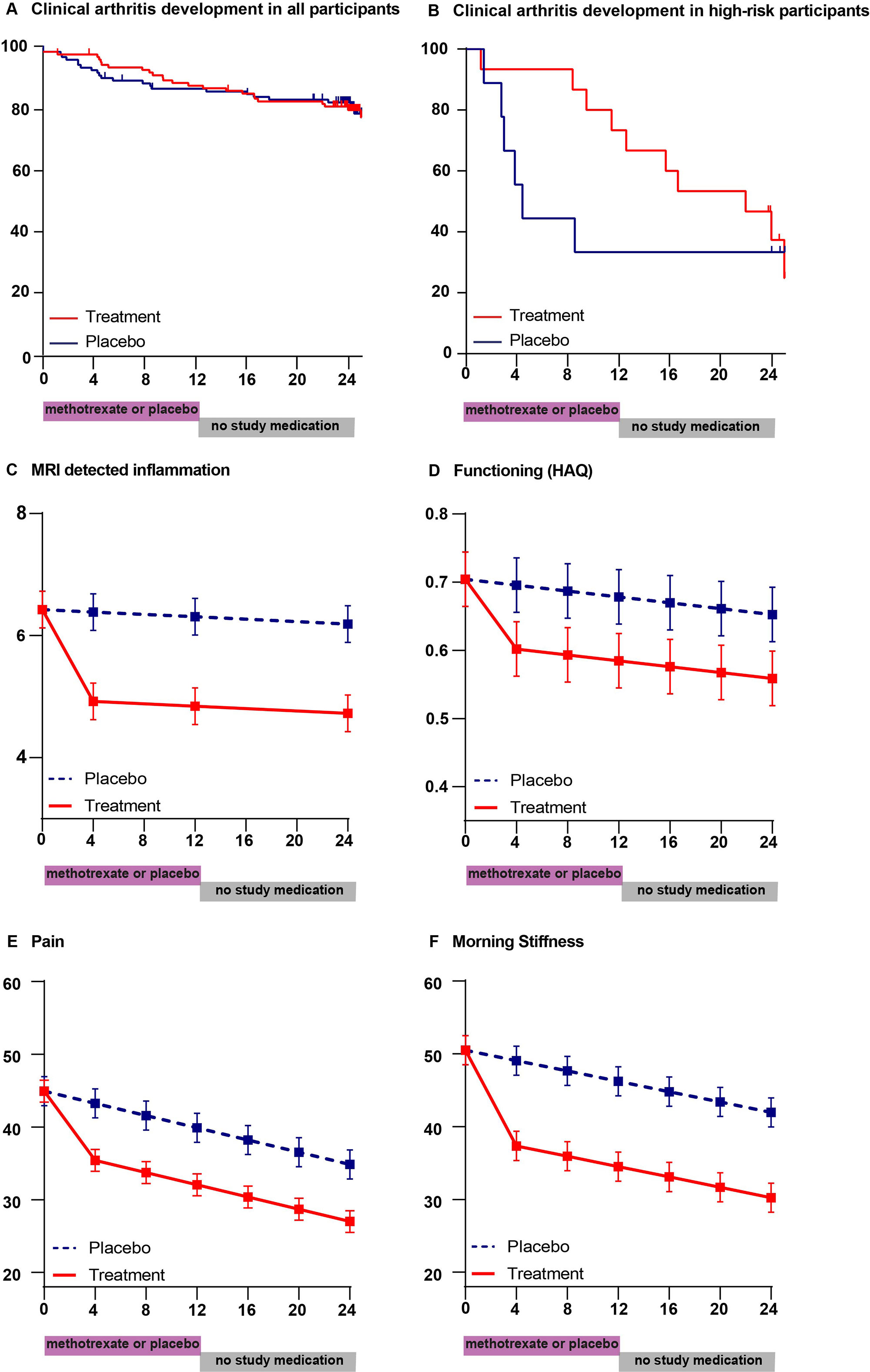
Results: From April 16th, 2015 to September 11th, 2019, we randomly assigned 236 participants to treatment (n=119) or placebo (n=117). After 24-months, arthritis-free survival was similar in both groups (81% versus 82%, HR 0.81 (95%CI 0.45,1.48)). Physical functioning improved more in the treatment-group during the first months and remained better (mean between-group difference over two-years HAQ -0.1 (-0.2,-0.03; p=0.004). Similarly, pain (scale 0-100: -9 (-12,-4; p< 0.001), morning stiffness (-12 (-16,-8; p< 0.001), presenteeism (-8% (-13%,-3%; p=0.001) and MRI-detected joint-inflammation (-1.4 points (-2.0,-0.9; p< 0.001) showed sustained improvement compared to placebo. High-risk participants in the treatment group developed less persistent clinical arthritis during treatment (HR at 12 months 0.26 (0.07; 0.93)), but frequencies became similar at 24 months (67% in both groups). A delaying effect was also present in ACPA-positive participants. Physical functioning, pain, presenteeism and MRI-detected inflammation showed sustained treatment induced improvements in these subgroups. The number of serious adverse events was equal between the treatment groups; adverse events were as expected from methotrexate.
Conclusion: Methotrexate, the cornerstone treatment of RA, initiated at the pre-arthritis stage of symptoms and subclinical inflammation, did not prevent the development of clinical arthritis, but modified disease course as measured by sustained improvement in MRI-detected inflammation, related symptoms and impairments.
To cite this abstract in AMA style:
Krijbolder D, Verstappen M, van Dijk B, Dakkak Y, Boer A, Park Y, de Witt-Luth M, Visser K, Kok M, Molenaar E, de Jong P, Böhringer S, Huizinga T, Allaart C, Niemantsverdriet E, van der Helm-van Mil A. Intervention with Methotrexate in Arthralgia at Risk for Rheumatoid Arthritis to Reduce the Development of Persistent Arthritis and Its Disease Burden (TREAT EARLIER): A Double-blind, Randomized, Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/intervention-with-methotrexate-in-arthralgia-at-risk-for-rheumatoid-arthritis-to-reduce-the-development-of-persistent-arthritis-and-its-disease-burden-treat-earlier-a-double-blind-randomized-plac/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intervention-with-methotrexate-in-arthralgia-at-risk-for-rheumatoid-arthritis-to-reduce-the-development-of-persistent-arthritis-and-its-disease-burden-treat-earlier-a-double-blind-randomized-plac/