Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Medication nonadherence is as high as 80% among SLE patients and leads to higher morbidity, mortality, and healthcare costs. Both the under-recognition of nonadherence by providers and patient-perceived poor communication with providers contribute to the problem. We developed and pilot tested an adherence intervention to reliably identify nonadherence and improve patient-provider communication regarding SLE medications. To conduct the intervention, the provider used commercial pharmacy refill data available in the electronic health records to monitor nonadherence and prompt a tailored discussion with the patient surrounding SLE medication use during the clinic encounter. In this analysis, we explored the effect of the intervention on adherence and differences between patients who did and did not benefit from the intervention.
Methods: The intervention was pilot tested over 12 weeks at an academic lupus clinic during regular follow-up visits. Consecutive patients on SLE medications, including HCQ, MTX, AZA, MMF, LEF, and belimumab, were included. Adherence was assessed by medication possession ratio (MPR) based on pharmacy refill data, using MPR ≥80% as a cutoff for adherence.
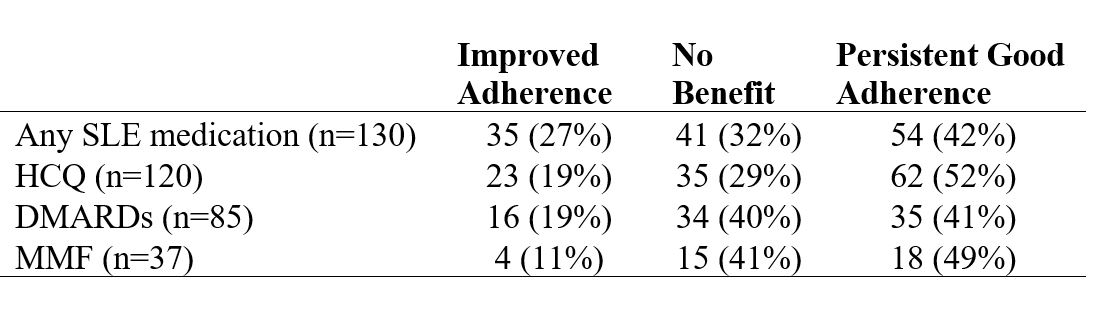
MPRs 3-months before (pre-) and after (post-) the intervention visit for each SLE medication were collected. Patients were grouped into 1) Improved Adherence (MPR < 80% pre-intervention but ≥80% post-intervention), 2) No Benefit from the intervention (MPR < 80% both pre- and post-intervention, or MPR ≥80% pre-intervention but < 80% post-intervention), and 3) Persistent Good Adherence (MPR ≥80% both pre- and post-intervention).
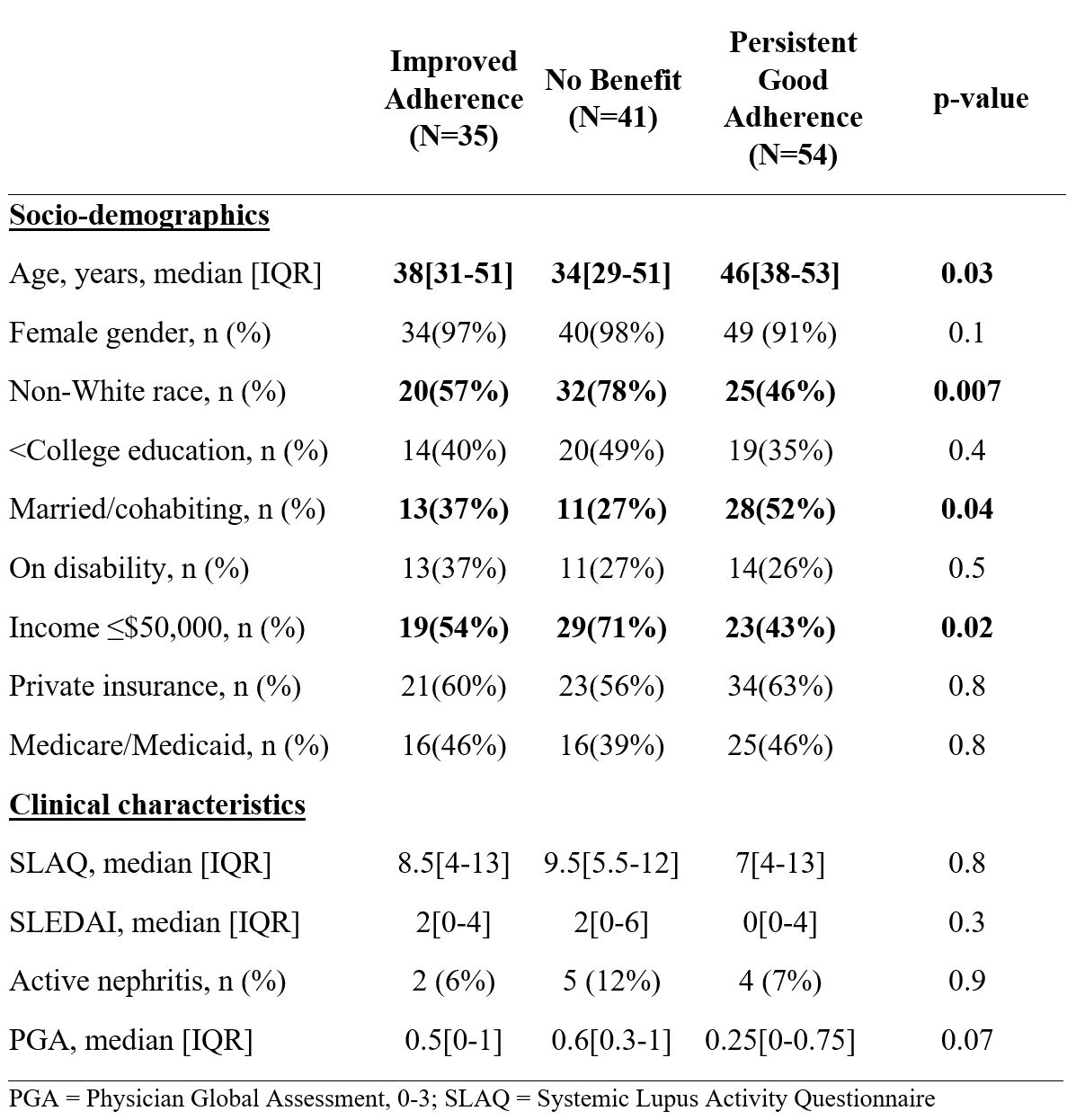
Results: Among 130 patients included in the analysis, median age was 43, 95% were female, 59% were non-White, and 40% were married or cohabiting. A significant proportion of patients had < college education (41%), were medically disabled (29%), and the majority had income < $50,000/year (55%). During the 3-month periods before and after the intervention visit, 92% patients were prescribed HCQ, 28% MMF, 22% AZA, 12% MTX, 7% belimumab, and 2% LEF. Most (59%) were on ≥2 SLE medications.
Improved Adherence was found in 27% of patients, while 32% had No Benefit and 42% had Persistent Good Adherence when considering all SLE medications. A notable proportion of patients experienced Improved Adherence when considering HCQ alone, MMF alone, or all DMARDs combined (Table 1). Compared to those who had No Benefit, those who had Improved Adherence after the intervention visit were more likely to be older, married or cohabitating, White, and have income >$50,000 (Table 2).
Conclusion: This simple intervention made routine use of a measurable, objective indication of adherence through pharmacy refill data and showed promising preliminary results in improving adherence in a significant proportion of SLE patients. However, additional research is needed to refine the intervention to best serve younger, non-White, and lower income patients, who often have more severe disease and are at highest risk for nonadherence.
To cite this abstract in AMA style:
Sun K, Eudy A, Rogers J, Sadun R, Criscione-Schreiber L, Doss J, Maheswaranathan M, Barr A, Eder L, Corneli A, Bosworth H, Clowse M. Intervention to Improve SLE Medication Adherence [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/intervention-to-improve-sle-medication-adherence/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intervention-to-improve-sle-medication-adherence/