Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In axial spondyloarthritis (axSpA), assessment of disease activity and physical function in clinical studies relies on patient-reported outcomes (PRO) such as patient’s global, BASDAI, ASDAS and BASFI. However, it still remains unclear how response criteria adequately reflects the patients’ opinion on disease status in daily practice. We aimed to investigate whether the results of PROs used in clinical studies with axSpA are indeed related to patient’s opinion on disease status as reported in daily clinical routine.
Methods: Data were retrieved from the very first and from the last five visits within a timeframe of 5 years. Patient´s clinical characteristics, physician’s global assessment and PROs (ASDAS, BASDAI, BASFI) were assessed at each visit. Status and change of all assessed information during follow-up were compared with patient´s opinion on symptoms related to axSpA, categorized into absent, mild, severe or very severe.
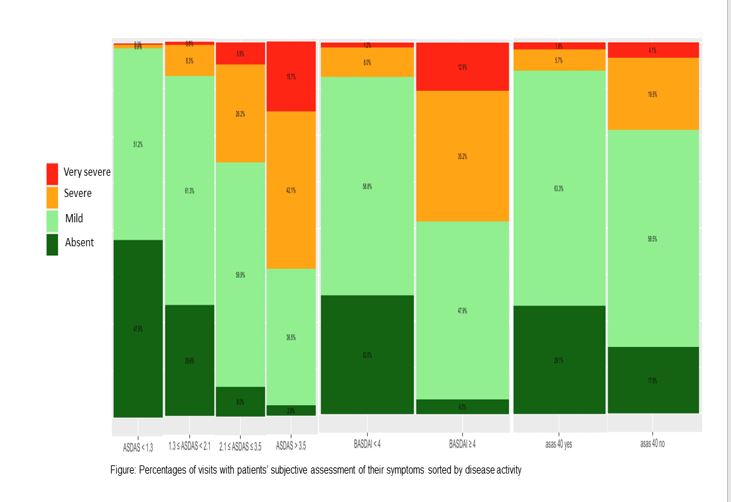
Results: 3,120 visits with median follow-up (IQR) 4.7 (4.3) years from 557 axSpA patients were analyzed. Mild symptoms were stated in 98.7% and 90.0% of visits with inactive or low ASDAS disease status, while 67.9% and 39.3% of visits showing association to high or very high ASDAS disease activity status, respectively (Fig.). In comparison, BASDAI < 4 was found in 90.8% of visits with mild symptoms, while BASDAI≥4 showed severe symptoms in 76.4% of visits (Fig.).
Achievement of ASAS40 was associated with 92.4% visits reporting absent/mild symptoms, while this was the case in 76.4% visits despite not reaching ASAS40. Similar data were observed for ASAS20. Severe symptoms were reported in 0.6% patients achieving vs. 30.1% patients not achieving ASAS partial remission (PR). BASFI correlated with patients’ opinion regarding symptoms at each visit.
Conclusion: Low disease activity as assessed by ASDAS or BASDAI were associated with mild symptoms in the majority of visits over a period of up to 5 years. Interestingly, a large proportion of visits showed low disease activity even when not achieving ASAS20 or ASAS40 responses or ASAS-PR.
To cite this abstract in AMA style:
Tsiami S, Klavdianou K, Redeker I, Özdemir Y, Sewerin P, Kiefer D, Andreica I, Kiltz U, Baraliakos X. Interpretation of Disease-specific Questionnaires on Disease Activity, Functional Capacity and Quality of Life in Daily Practice in Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/interpretation-of-disease-specific-questionnaires-on-disease-activity-functional-capacity-and-quality-of-life-in-daily-practice-in-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interpretation-of-disease-specific-questionnaires-on-disease-activity-functional-capacity-and-quality-of-life-in-daily-practice-in-axial-spondyloarthritis/