Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Little is known regarding differences in DMARD utilization across countries. A better understanding is needed to contextualize findings from different countries.1 Using the same type of registry and design approach, our study describes baseline characteristics and clinical measures among rheumatoid arthritis (RA) patients who initiated a disease-modifying anti-rheumatic drug (DMARD) in Japan and the US.
Methods: We identified RA patients in the Corrona RA Japan (RA-J) and Corrona RA US (RA-US) Registries who initiated a DMARD (methotrexate, TNF, nonTNF, Janus kinase inhibitor (JAKi)) between 01/March/2016 to 31/January/2020. We compared cohorts overall and by drug group for sociodemographics, disease characteristics, comorbidities, patient-reported outcome measures (PROMs), and medications (current and prior). Of note, 2nd, 3rd, and 4th DMARDs were initiated later in RA-J in terms of disease duration. Descriptive statistics were provided; t-tests were used for continuous variables, chi-squared tests for categorical or dichotomous variables, and Fisher’s exact tests for categorical/dichotomous variables with a category size smaller than five.
Results: Baseline data were available for 1996 RA-J and 6846 RA-US drug initiators. Overall, the two cohorts differed in most of the variables except that both groups were 79% female. At baseline, RA-J vs RA-US had a shorter mean duration of RA disease (8.1 yrs vs 10.4 yrs), higher percentage of history of serious infections (12% vs 9%), higher level of disease activity (CDAI: 22.5 vs 18.8), swollen joint count (6.2 vs 4.6), and physician global assessment (45.5 vs 31.7), but a lower percentage of history of cardiovascular disease (11% vs 14%) (Table 1).
When RA-J vs RA-US were stratified by drug class, the pattern of the differences between the two cohorts was mostly consistent with the overall analysis, except that the RA-J MTX initiators had a lower level of CDAI (19.1 vs 21.2) and prednisone use (17% vs 37%) than RA-US MTX initiators, while among the other groups (TNFi, nonTNFi, and JAKi) the pattern was reverse for CDAI or not present for prednisone use (Table 2).
Disease activity, PROMs, and current medication use was different between the two cohorts by line of therapy. Among RA-J vs RA-US, mean pain scores were higher (52.9 vs 45.9) in the 3rd line of therapy, and mean CDAI scores were higher [(21.8 vs 17.7), (22.3 vs 16.1), (25.1 vs 19.8)] and more prednisone use occurred [(30% vs 26%), (33% vs 26%), (37% vs 33%)] in the 2nd, 3rd, and 4th lines of therapy, respectively. A greater percentage of RA-J vs RA-US used nonTNFi in the 1st (12% vs 3%), 2nd (41% vs 10%), and 3rd line (40% vs 22%) of therapy. By contrast in RA-J vs RA-US, TNFi use was lower in the 1st (4% vs 8%), 2nd (40% vs. 63%), 3rd (32% vs 60%) and 4th line (23% vs 36%) of therapy.
Conclusion: Patients in RA Japan and RA US Registries differed in many aspects of demographics, comorbidity histories, clinical measures, and the utilization of DMARDs. Understanding cross-cultural differences in RA management is essential when interpreting results of studies from different countries.
Verstappen SM, et al. Arthritis Care Res (Hoboken). 2015 Dec;67(12):1637-45.
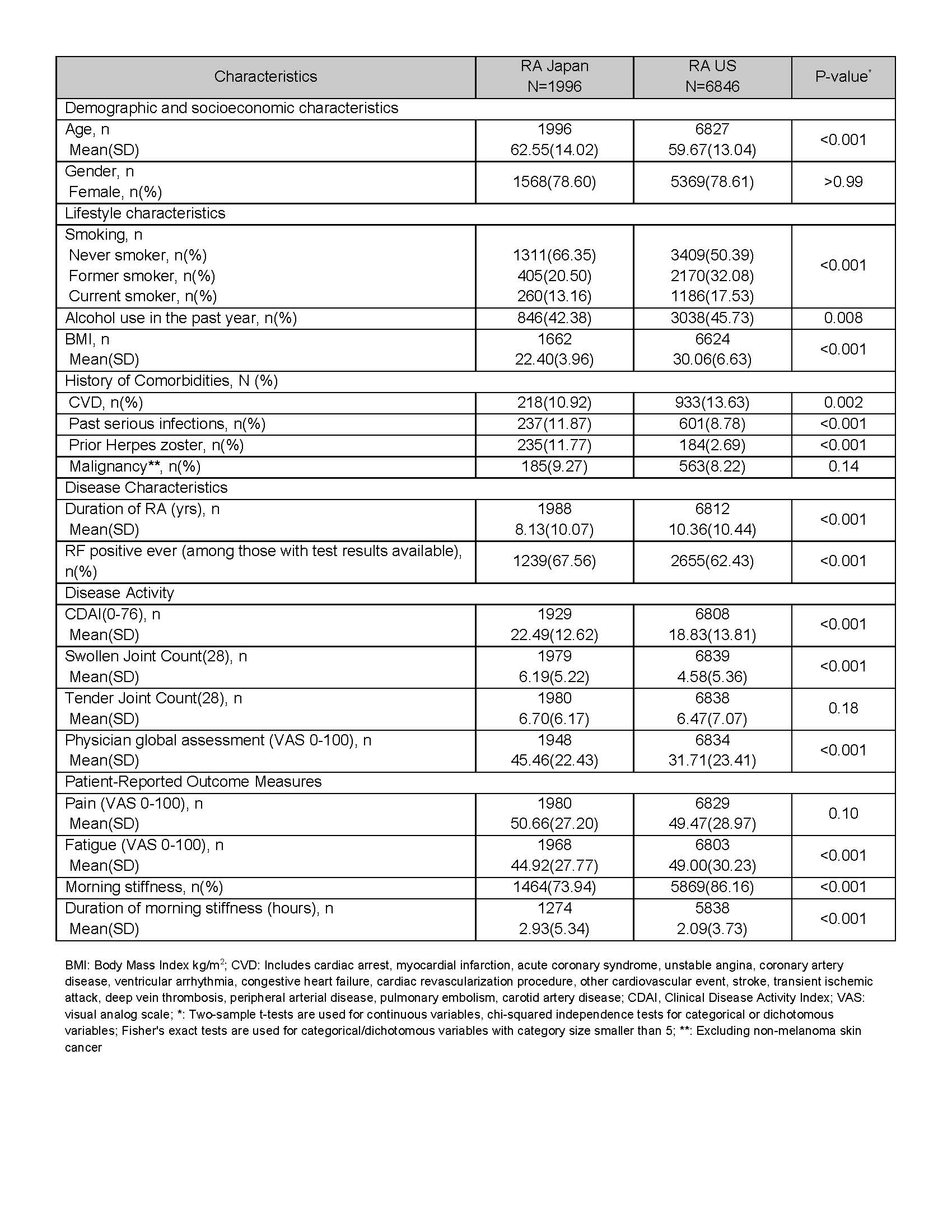 Table 1. Baseline Characteristics of RA Japan and RA US Drug Initiators, Overall
Table 1. Baseline Characteristics of RA Japan and RA US Drug Initiators, Overall
 Table 2. Baseline characteristics of RA Japan and RA US, by drug class
Table 2. Baseline characteristics of RA Japan and RA US, by drug class
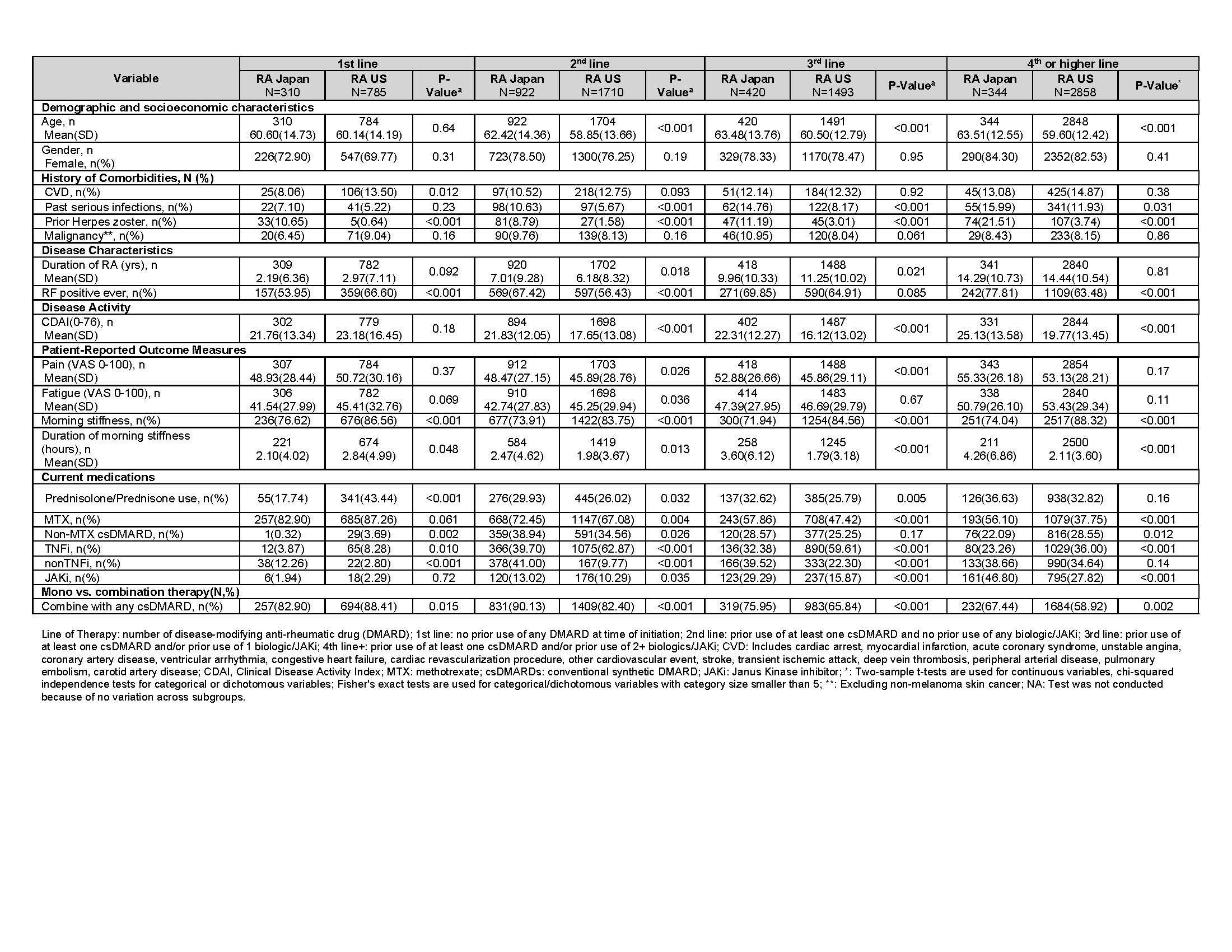 Table 3. Baseline characteristics of RA Japan and RA US drug initiators, stratified by line of therapy
Table 3. Baseline characteristics of RA Japan and RA US drug initiators, stratified by line of therapy
To cite this abstract in AMA style:
Yamanaka H, Kishimoto M, Nakano K, Misaki K, Yamanishi Y, Dobashi H, Natsumeda M, Miyamoto T, Amano K, Sagawa A, Koido N, Consortium C, Harrold L, Lin T, Greenberg J, Tanaka Y. International Comparison of Japanese and US Cross Country Utilization of RA Medications [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/international-comparison-of-japanese-and-us-cross-country-utilization-of-ra-medications/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/international-comparison-of-japanese-and-us-cross-country-utilization-of-ra-medications/
