Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Lesinurad is a selective uric acid reabsorption inhibitor recently approved at 200 mg daily in combination with a xanthine oxidase inhibitor (XOI) for treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid on XOI (allopurinol or febuxostat) alone. We integrated safety data for lesinurad (LESU) based on: (1) 3 large, pivotal, placebo-controlled, 12-month phase III (core) trials evaluating LESU 200 mg and LESU 400 mg in combination with an XOI; and (2) 2 extension studies, in which LESU-treated patients continued to receive LESU + XOI at the same dose and initially placebo-treated patients were randomized to receive LESU 200 mg or LESU 400 mg in addition to the XOI provided in the preceding core trial.
Methods: Safety data were pooled from the 3 core studies and 12-month extension studies using descriptive statistics for patients receiving ≥1 dose of study medication. To adjust for varying treatment durations, treatment-emergent adverse events (TEAEs) are expressed as exposure-adjusted incidence rates (subjects with events per 100 person-years [PY]).
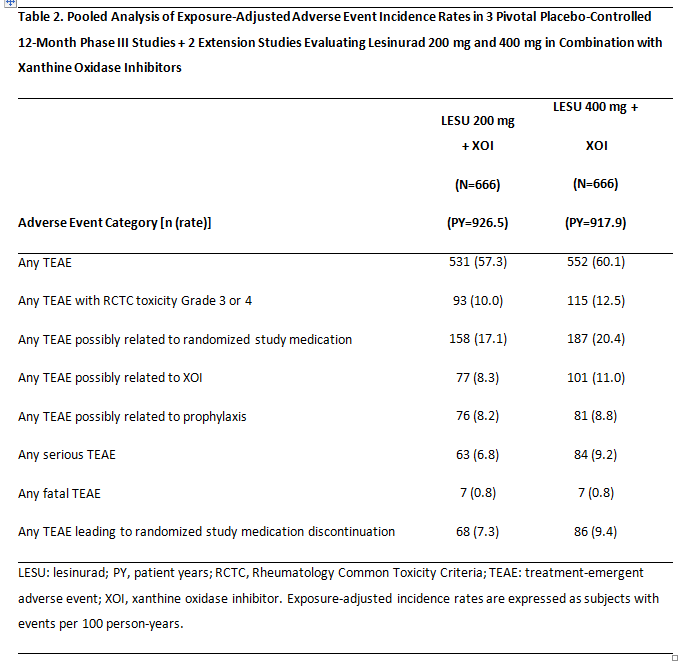
Results: In the core studies, adverse event rates were comparable for XOI alone and LESU 200 mg + XOI groups for any TEAEs, serious TEAEs, and TEAEs leading to discontinuation (Table 1). Adverse event rates were higher with LESU 400 mg + XOI. Major adverse cardiovascular event (MACE) rates, which included cardiovascular death, myocardial infarction, or stroke, in the core studies were 0.71 (95% CI 0.15, 2.08), 0.96 (0.26, 2.47), and 1.94 (0.84, 3.82) per 100 PY for XOI alone, LESU 200 mg + XOI, and LESU 400 mg + XOI, respectively. Renal-related TEAE rates in the core studies were 5.6, 7.3, and 15.4 per 100 PY, respectively. Longer exposure in the core + extension studies did not result in increases in any TEAEs, serious TEAEs, or TEAEs leading to discontinuation (Table 2). MACE rates were low in the core + extension studies, at 1.05 (95% CI 0.50, 1.93) and 1.48 (0.81, 2.48) per 100 PY in the LESU 200 mg + XOI and LESU 400 mg + XOI groups, respectively. Renal events in the core + extension studies were lower in the LESU 200 mg + XOI than LESU 400 mg + XOI group at 8.6 and 14.6 per 100 PY, respectively.
Conclusion: Lesinurad at the approved dose of 200 mg once-daily combined with XOI demonstrated a consistent, acceptable safety profile. There were no new safety concerns in the extension studies. 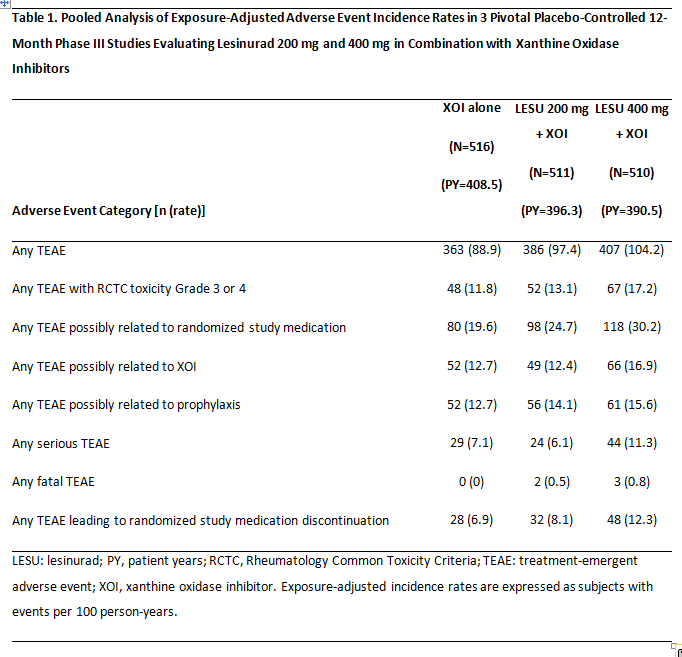
To cite this abstract in AMA style:
Becker MA, Keenan RT, Khanna P, Malamet R, Bos K, Li J, Hu J, White W. Integrated Safety of Lesinurad, a Novel Uric Acid Reabsorption Inhibitor for the Treatment of Gout [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/integrated-safety-of-lesinurad-a-novel-uric-acid-reabsorption-inhibitor-for-the-treatment-of-gout/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-safety-of-lesinurad-a-novel-uric-acid-reabsorption-inhibitor-for-the-treatment-of-gout/