Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Upadacitinib (UPA) is an oral Janus kinase inhibitor approved for the treatment of rheumatoid arthritis (RA). The safety and efficacy of UPA has been evaluated across a spectrum of patients (pts) with RA in the Phase 3 SELECT clinical program.1,2
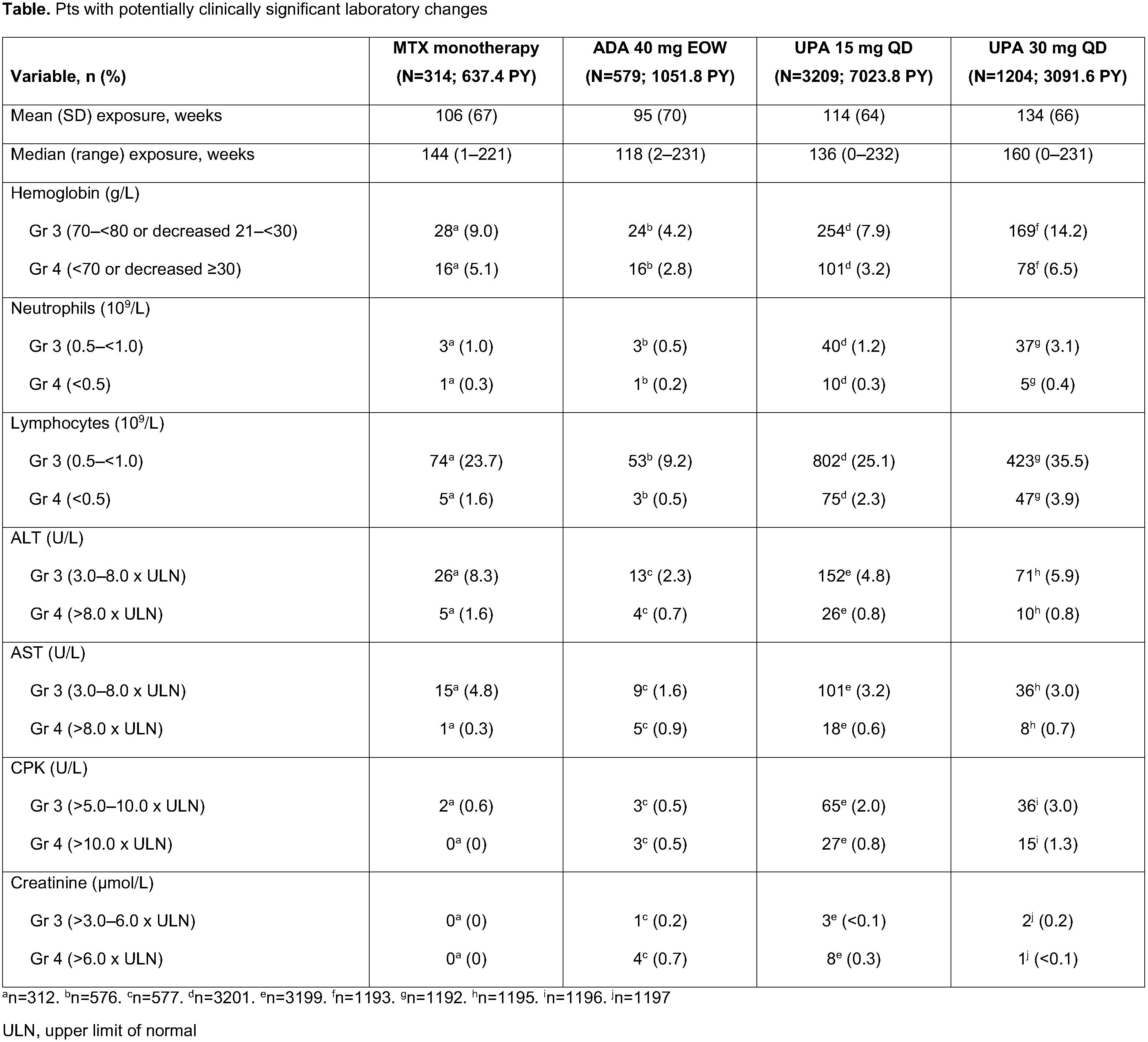
We describe long-term laboratory profiles (cut-off date: Jun 30, 2020) associated with exposure to UPA, adalimumab (ADA), and methotrexate (MTX) in pts with RA treated in the SELECT trials.
Methods: Data were analyzed from 6 randomized controlled UPA RA trials.1,2 The proportions of pts experiencing potentially clinically significant laboratory changes at a single time point were summarized for the following groups: pooled UPA 15 mg once daily (QD; UPA15; 6 trials), pooled UPA 30 mg QD (UPA30; 4 trials), ADA 40 mg every other week (EOW; 1 trial), and MTX monotherapy (1 trial). Pts received UPA with/without background conventional synthetic disease-modifying antirheumatic drugs. Treatment-emergent adverse events are reported as exposure-adjusted event rates (events/100 patient years [E/100 PY]). Toxicity was graded per OMERACT criteria, or NCI CTCAE for creatine phosphokinase (CPK) and creatinine.
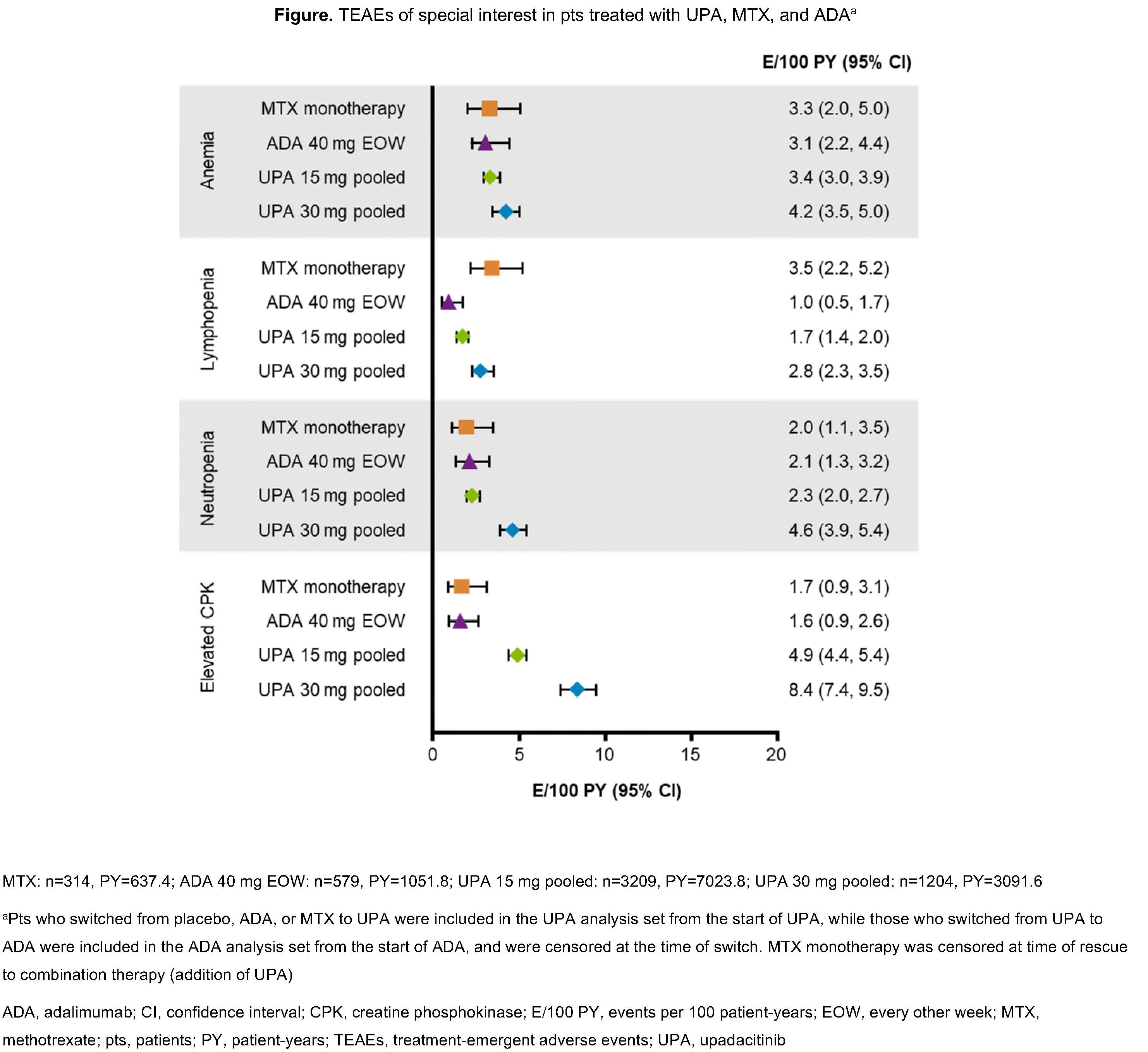
Results: 4413 pts received ≥1 dose of UPA (UPA15 [n=3209]; UPA30 [n=1204]). Exposures were comparable between treatment groups (Table). Proportions of pts with Grade (Gr) 3 and 4 decreases in hemoglobin were highest with UPA30 and MTX (Table). Rates of anemia, as reported by the investigator, were comparable between UPA15, ADA, and MTX groups (Figure); the frequency of UPA-treated pts who discontinued due to anemia was uncommon in all arms. Gr 3 and 4 decreases in neutrophil and lymphocyte counts with UPA were dose-dependent and higher vs ADA or MTX. Discontinuations due to neutropenia and lymphopenia were rare (< 0.1%). Transaminase elevations were more frequent with UPA and MTX vs ADA; however, the proportion of pts who discontinued due to increases in alanine (ALT) or aspartate aminotransferase (AST) were comparable between UPA15 and ADA, and numerically higher with UPA30 and MTX. CPK elevations were more frequent with UPA (Figure). Most events were asymptomatic, and the 1 case of rhabdomyolysis in the UPA30 group was unrelated to study drug (which was attributed to influenza).
Conclusion: This long-term analysis of UPA-treated pts with RA showed dose-dependent relationships for several laboratory abnormalities. Incidences of these with UPA15 were similar to MTX but typically higher than with ADA. Treatment discontinuations due to laboratory abnormalities were infrequent and similar across all treatment groups.
1. Tanaka Y. Mod Rheumatol 2020;30:779–87; 2. Rubbert-Roth A, et al. N Engl J Med 2020;383:1511–21
To cite this abstract in AMA style:
Charles-Schoeman C, Giles J, Lane N, Choy E, Furst D, Vencovsky J, Wilson A, Burmester G, Shaw T, Song Y, Camp H, Khan N, Yee J, Anyanwu S, McInnes I. Integrated Laboratory Abnormality Profiles of Upadacitinib with up to 4.5 Years of Exposure in Patients with Rheumatoid Arthritis Treated in a Phase 3 Clinical Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/integrated-laboratory-abnormality-profiles-of-upadacitinib-with-up-to-4-5-years-of-exposure-in-patients-with-rheumatoid-arthritis-treated-in-a-phase-3-clinical-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-laboratory-abnormality-profiles-of-upadacitinib-with-up-to-4-5-years-of-exposure-in-patients-with-rheumatoid-arthritis-treated-in-a-phase-3-clinical-trial/