Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Long‑term prevention of structural joint damage is a key treatment goal in the management of RA1. Upadacitinib (UPA), a JAK1-selective inhibitor, inhibited the progression of structural joint damage at 6 months as monotherapy in methotrexate (MTX)‑naïve RA patients (pts)2 and in combination with MTX in pts with inadequate response (IR) to MTX3.
We evaluate the progression of structural joint damage (radiographic) through Week 48 in pts with moderately to severely active RA treated with UPA monotherapy or in combination with MTX.
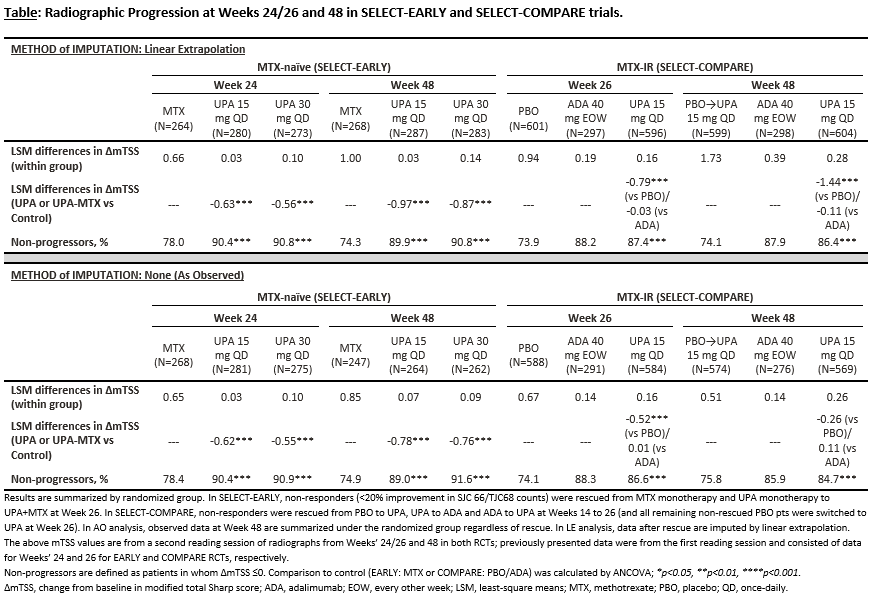
Methods: Radiographic progression was assessed in 2 phase 3 randomized controlled trials (RCTs), as previously described2,3. MTX‑naïve pts were randomized to UPA 15 or 30mg QD or MTX monotherapy [SELECT‑EARLY, N=945], while MTX-IR pts were randomized to UPA 15mg QD or adalimumab (ADA) 40 mg eow or placebo (PBO), with continuous background MTX [SELECT‑COMPARE, N=1629]. Both RCTs specifically enrolled pts at high risk for progression of joint damage (high disease activity including elevated hsCRP, presence of baseline erosions and ACPA and/or RF positivity2,3). The mean changes (Δ) from baseline (BL) in modified Total Sharp Score (mTSS), joint space narrowing (JSN), and erosion scores (ES) as well as the proportion of pts with no radiographic progression (∆mTSS ≤0) at Weeks 24/26 and 48 were determined in both RCTs. Data were analyzed by linear extrapolation (LE) for missing data imputation and treatment switching, and as observed (AO).
Results: BL demographics have been reported previously2,3. At Weeks 24/26, UPA as monotherapy and in combination with background MTX significantly inhibited radiographic progression measured by mean ΔmTSS and the proportion of pts with no radiographic progression vs MTX and PBO, respectively (LE and AO, Table). The significant inhibition of radiographic progression with UPA was maintained through Week 48 vs MTX (LE and AO) in EARLY and vs PBO (LE) in COMPARE. Following the switch of all PBO pts to UPA in COMPARE by Week 26, no further change in mean mTSS was observed through Week 48 (AO, Table). The inhibition of radiographic progression vs comparators was not only observed for the overall mTSS scores but also its components – the JSN and ES in both RCTs (LE and AO).
Conclusion: UPA both as monotherapy, and in combination with background MTX, was effective in inhibiting the progression of structural joint damage through Week 48 in MTX-naïve, and MTX-IR patients, respectively.
1. Smolen JS et al. Ann Rheum Dis 2017;0: 1–18
2. van Vollenhoven R et al. Arthritis Rheumatol. 2018; 70 -suppl 10- [ACR 2018 abstract]
3. Fleischmann R et al. Arthritis Rheumatol. 2018; 70 -suppl 10- [ACR 2018 abstract]
To cite this abstract in AMA style:
Peterfy C, Genovese M, Song I, Friedman A, Hall S, Mysler E, Durez P, Baraliakos X, Enejosa J, Shaw T, Li Y, Chen S, Strand V. Inhibition of Structural Joint Damage with Upadacitinib as Monotherapy or in Combination with Methotrexate in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/inhibition-of-structural-joint-damage-with-upadacitinib-as-monotherapy-or-in-combination-with-methotrexate-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-structural-joint-damage-with-upadacitinib-as-monotherapy-or-in-combination-with-methotrexate-in-patients-with-rheumatoid-arthritis/