Session Information
Date: Monday, November 9, 2015
Title: Systemic Sclerosis, Fibrosing Syndromes and Raynaud's - Pathogenesis, Animal Models and Genetics I
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Systemic sclerosis (SSc) is a
progressive, debilitating disease with limited treatment options. IL-6 has been
implicated in disease pathogenesis. Tocilizumab (TCZ), an IL-6Rα inhibitor,
was evaluated in the 2-year faSScinate study, a randomized, double-blind,
placebo-controlled trial. At week 24 (primary end point), favorable trends in
skin score for TCZ were detected though the primary skin score end point was
not met. In addition, smaller declines in FVC were observed in the TCZ-treated
patients.
Methods: Eighty-seven patients with active SSc
were randomly assigned 1:1 to subcutaneous TCZ 162 mg or placebo (PBO) weekly
for 48 weeks. The primary end point was mean change in modified Rodnan skin
score from baseline at week 24. Gene expression analysis was performed on skin biopsy
samples collected at baseline and week 24. First, genomewide expression
analysis was conducted on all available biopsy samples and on biopsy samples of
age- and sex-matched healthy volunteers (HVs) using custom Agilent 60-mer
microarray (Epistem, Manchester, UK). Based on these data, 86 genes
representing fibrosis, IFN, and myeloid pathways were selected for more
quantitative gene expression analysis using nCounter technology (NanoString Technologies,
Seattle, WA, USA). CCL18 serum levels were determined using an IMPACT-based
immunoassay (Roche Diagnostics, Penzberg, Germany).
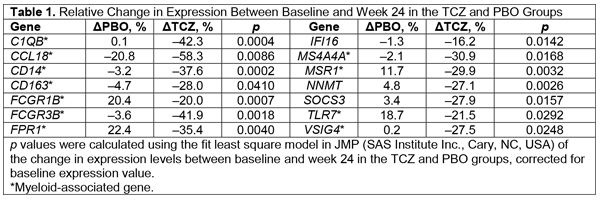
Results: Of the 86 genes selected for follow-up
expression analysis, 75 were, on average, significantly overexpressed in SSc
patients compared with HVs. Analysis of genes significantly downregulated after
TCZ treatment and stable or increased with PBO identified a subset of 14 genes
highly enriched for myeloid-associated genes, including genes associated with
M2 macrophages (Table 1). All 14 genes were overexpressed in SSc patients
compared with HVs. No effect of TCZ on the fibrosis and IFN pathways was
detected. Serum levels of the M2 macrophage chemokine CCL18 revealed a rapid and
sustained decrease from elevated levels in the TCZ group to close to levels in
healthy controls but not in PBO controls.
Conclusion: The effect of TCZ on
myeloid-associated genes may reflect inhibition and/or depletion of
skin-infiltrating macrophages. In addition, the effect of TCZ on CCL18 (RNA and
protein), CD163, MS4A4A, and MSR1 suggests a specific inhibitory effect of TCZ
on M2 macrophages, which are known to promote fibrosis and inflammation. These
findings represent a potential novel mode of action for TCZ in SSc, which will
be further investigated in the upcoming phase 3 study of TCZ in SSc.
To cite this abstract in AMA style:
Sornasse T, Chen H, Rice L, Stifano G, Jahreis A, Siegel J, Lafyatis R. Inhibition of Myeloid-Associated Gene Expression in Skin Biopsy Samples of Systemic Sclerosis Patients Treated with Tocilizumab [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/inhibition-of-myeloid-associated-gene-expression-in-skin-biopsy-samples-of-systemic-sclerosis-patients-treated-with-tocilizumab/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-myeloid-associated-gene-expression-in-skin-biopsy-samples-of-systemic-sclerosis-patients-treated-with-tocilizumab/