Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Peficitinib (PEF), a novel oral Janus kinase (JAK) inhibitor, has demonstrated efficacy in Phase 3 studies of patients with RA (NCT02305849).1 We report the evaluation for suppression of joint destruction of PEF compared with placebo (PBO) in this study.
Methods: This randomized, double-blind, PBO-controlled study was conducted in Japan. Patients with RA (< 10 years’ duration) and inadequate response (IR) to MTX were randomly assigned 1:1:1 to receive PEF 100 or 150 mg/day (PEF100 or PEF150) or PBO once daily, plus MTX. Patients receiving PBO were switched to PEF100 or PEF150 at Week (W) 28, or W12 for IR. Hand and foot X-rays were taken at baseline (BL), W12, 28 and 52. Mean change from BL (linear extrapolation) in van der Heijde modified Total Sharp Score (mTSS) at W28 in the full analysis set (FAS) was a primary endpoint, and was analyzed with rank analysis of covariance (rank ANCOVA) with treatment group as factor and BL rank mTSS as covariate in the primary analysis. Sensitivity analyses of the primary efficacy analysis were: 1. rank ANCOVA with LOCF imputation; 2. rank ANCOVA as observed data; 3. rank ANCOVA using the per protocol set-mTSS as the analysis set; 4. ANCOVA; 5. ANCOVA with multiple imputation method. The proportions of patients with no joint damage (mTSS < 0.5) and yearly progression of mTSS >5 were calculated. Subgroup analyses of mTSS for different demographic and BL characteristics were presented, in which interaction term with treatment group was significant at a two-sided significance level of 0.15 by ad hoc analysis.
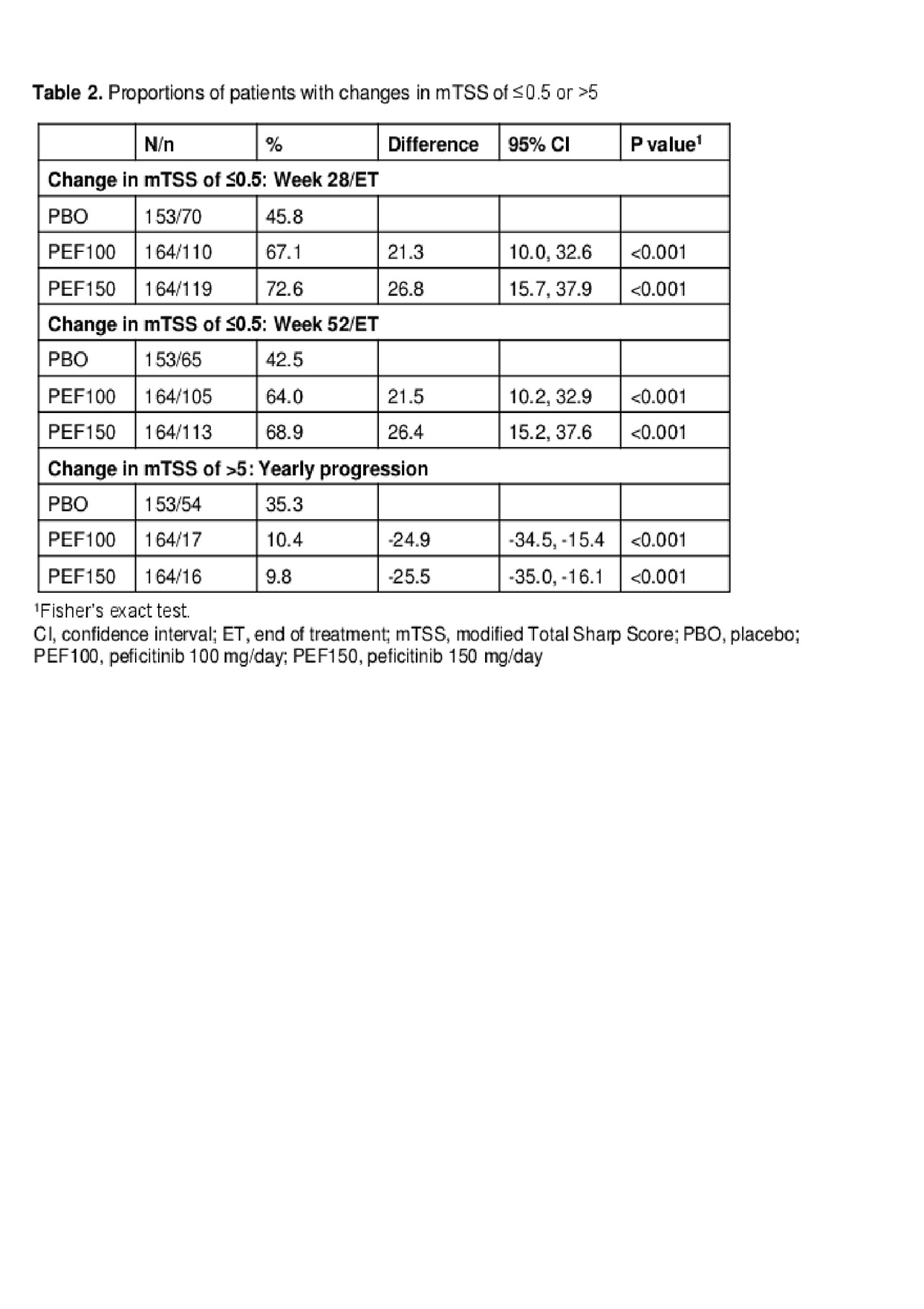
Results: In total, 518 patients were included in the FAS: PBO n=170; PEF100 n=174; PEF150 n=174. BL demographics were similar between groups. The primary and secondary endpoint results from RAJ4 were met and have been described previously.1 Sensitivity analyses showed that the change in mTSS at W28 was similar to the primary analysis result, showing the robustness of the primary analysis (Table 1). At W28, a significantly greater proportion of patients achieved a change in mTSS of ≤0.5 for both PEF100 and PEF150 compared with PBO (67.1% and 72.6% vs 45.8%, respectively; p< 0.001 for both). This was maintained at W52 (64.0% and 68.9% vs 42.5%, respectively; p< 0.001 for both) (Table 2). The proportion of patients showing rapid radiographic progression (yearly progression of mTSS ≥5) was also significantly lower for both PEF100 and PEF150 compared with PBO (10.4% and 9.8% vs 35.3%, respectively; p< 0.001 for both: Table 2).
Selected parameters from the subgroup analysis showing significant results for their interaction with treatment group were age group, BL mTSS, BL DAS28-CRP, duration of RA, BL CRP, concomitant steroid at BL, prednisone dose at BL, and body weight at screening at a two-sided significance level of 0.15 by ad hoc analysis (Table 3).
Conclusion: Peficitinib 100 mg and 150 mg both demonstrated significant inhibition of joint destruction compared with PBO.
- T. Takeuchi et al. Arthritis Rheumatol 2018; 70 (Suppl 10): Abstract 888
To cite this abstract in AMA style:
Takeuchi T, Tanaka Y, Rokuda M, Izutsu H, Kaneko Y, Fukuda M, Kato D, van der Heijde D. Inhibition of Joint Destruction in Patients with Rheumatoid Arthritis Treated with Peficitinib in Combination with Methotrexate: A Randomized, Double-Blind, Placebo-Controlled Trial in Japan [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/inhibition-of-joint-destruction-in-patients-with-rheumatoid-arthritis-treated-with-peficitinib-in-combination-with-methotrexate-a-randomized-double-blind-placebo-controlled-trial-in-japan/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-joint-destruction-in-patients-with-rheumatoid-arthritis-treated-with-peficitinib-in-combination-with-methotrexate-a-randomized-double-blind-placebo-controlled-trial-in-japan/