Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Many pediatric rheumatology patients are at increased risk of influenza due to immunosuppressive medication use. Annual influenza vaccination is recommended for all children by the Centers for Disease Control and Prevention. Our division has tracked influenza vaccination rates since 2015 and have done quality improvement efforts to increase our vaccination rate. The vaccination rate plateaued at 71%, which prompted a survey to assess patient-reported vaccination rate, knowledge of influenza, and barriers to influenza vaccination.
Methods: In the Rheumatology Clinic, a parent or patient (15 years and older) on immunosuppressive medication was eligible to complete a REDCap survey. Survey questions assessed demographics, rheumatology diagnosis, immunosuppressive medication type, medical providers recommendation of the influenza vaccination, influenza knowledge, and barriers to influenza vaccination. Self-reported vaccination rate referred to the 2018-2019 influenza season; if no vaccine received, a follow-up question asked for reasons why. The survey began in July 2019, and this project is ongoing. Influenza vaccination rates of immunosuppressed patients are acquired each flu season via chart review and tracked on a control chart that our division has maintained since 2016.
Results: Of 212 surveys, 139 (65.6%) were completed by parents and 73 (34.4%) by patients. The patients had an average age of 11.4 years for those under 17 years of age, and 13.2% of respondents were 18 years or older. The majority (61.3%) were diagnosed with juvenile idiopathic arthritis, while 8.5% had juvenile dermatomyositis and 8% had systemic lupus erythematosus. Methotrexate was the most common (52.4%) immunosuppressive medication followed by a tumor necrosis factor inhibitor (41%). The majority (84.9%) of parents reported their child received an influenza vaccine while patient report was 80.8%. The most common reasons for not receiving the influenza vaccine included: worry about disease flare (28.6%), concern the vaccine will cause influenza (25.7%), and presumed lack of vaccine effectiveness (20%) (Table 1). When asked for the two most common symptoms of influenza, 82.5% correctly answered fever and 73.5% correctly answered cough and/or congestion; however, 23.1% answered gastrointestinal symptoms and 9.6% joint swelling. Almost all the respondents (95.3%) were aware that influenza could cause death and that the prescribed medication increased risk of infection. The average weekly influenza vaccination rate from October to December 2019 was 76.6%, which was higher than the 72.1% vaccination rate from the 2018-2019 season (Figure 1).
Conclusion: The influenza survey of immunosuppressed patients indicates that patients and parents understand the potential severity of influenza and the increased risk of infection due to medication use, however, many respondents inaccurately identified the most common symptoms of influenza. Additionally, self-reported vaccination rate was higher than expected. The barriers identified in this survey will help drive further improvement efforts to increase influenza vaccination rates in this high-risk population.
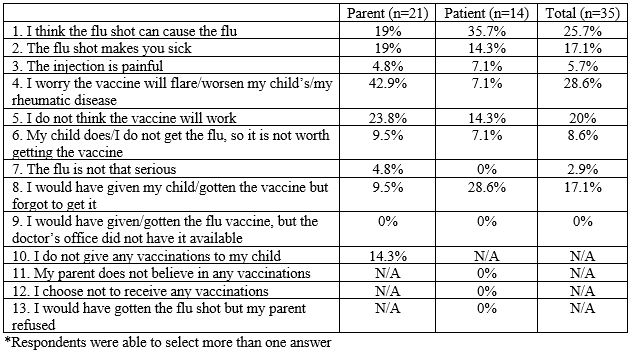 Table 1. Reasons why influenza vaccine not received*
Table 1. Reasons why influenza vaccine not received*
 Figure 1. Control chart demonstrating influenza vaccination rates in immunosuppressed rheumatology patients
Figure 1. Control chart demonstrating influenza vaccination rates in immunosuppressed rheumatology patients
To cite this abstract in AMA style:
Harris J, Ibarra M, Holland M, Jensen K, Fox E, Jones J, Favier L, Sherman A, Smith C, Cooper A. Influenza Knowledge and Barriers to Vaccination in Immunosuppressed Patients in the Pediatric Rheumatology Clinic [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/influenza-knowledge-and-barriers-to-vaccination-in-immunosuppressed-patients-in-the-pediatric-rheumatology-clinic-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/influenza-knowledge-and-barriers-to-vaccination-in-immunosuppressed-patients-in-the-pediatric-rheumatology-clinic-2/
