Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Dupilumab is a monoclonal antibody directed against the alpha subunit of the interleukin (IL)-4 receptor, blocking interleukin (IL)-4 and IL-13 signaling. It is approved for moderate-to-severe atopic dermatitis, moderate-to-severe asthma, nasosinusal polyposis, eosinophilic esophagitis and nodular prurigo. Cases of de novo or relapse of inflammatory diseases under treatment with dupilumab have been described following its commercialization. The aim of this study is to describe systemic inflammatory diseases occurring with dupilumab.
Methods: We conducted a retrospective, multicenter study using a call to collect observations from patients with systemic inflammatory disease who developed or relapsed after initiation of dupilumab. We then conducted a pharmacovigilance study using the Vigibase database to confirm or refute specific pharmacovigilance signals. We examined the reporting odds ratios (ROR), considering those with an ROR greater than 1 to be pharmacovigilance signals.
Results: We collected 20 case reports of inflammatory diseases that occurred on dupilumab (median age 62.5 years (IQR 53-70.75), males 40%, females 60%). Only one of the 20 cases was a relapse (eosinophilic granulomatosis with polyangiitis) of systemic disease, the others were newly diagnosed. These cases were: eosinophilic granulomatosis with polyangiitis (EGPA, n=7), giant cell arteritis (n=5), granulomatosis (n=2, one cutaneous granulomatosis and one sarcoidosis), two seronegative polyarthritis, and one psoriatic arthritis, Addison’s disease, immune thrombocytopenic purpura, and eosinophilic vasculitis in one case each. The median time to disease onset following initiation of dupilumab therapy was 5.5 months (IQR 3-23.25). Median blood eosinophilia at the time of inflammatory disease diagnosis was 1680/mm3 (IQR 1150-1650).
The occurrence of these diseases led to the discontinuation of dupilumab in 17 (85%). Oral glucocorticoids alone were initiated in 13 cases (65%), and in 5 cases treatment was based on glucocorticoids in combination with immunosuppressive or immunomodulatory agents (rituximab in 2, mepolizumab in 2, benralizumab in 1, and methotrexate in 1). One case of sarcoidosis regressed spontaneously without stopping dupilumab.
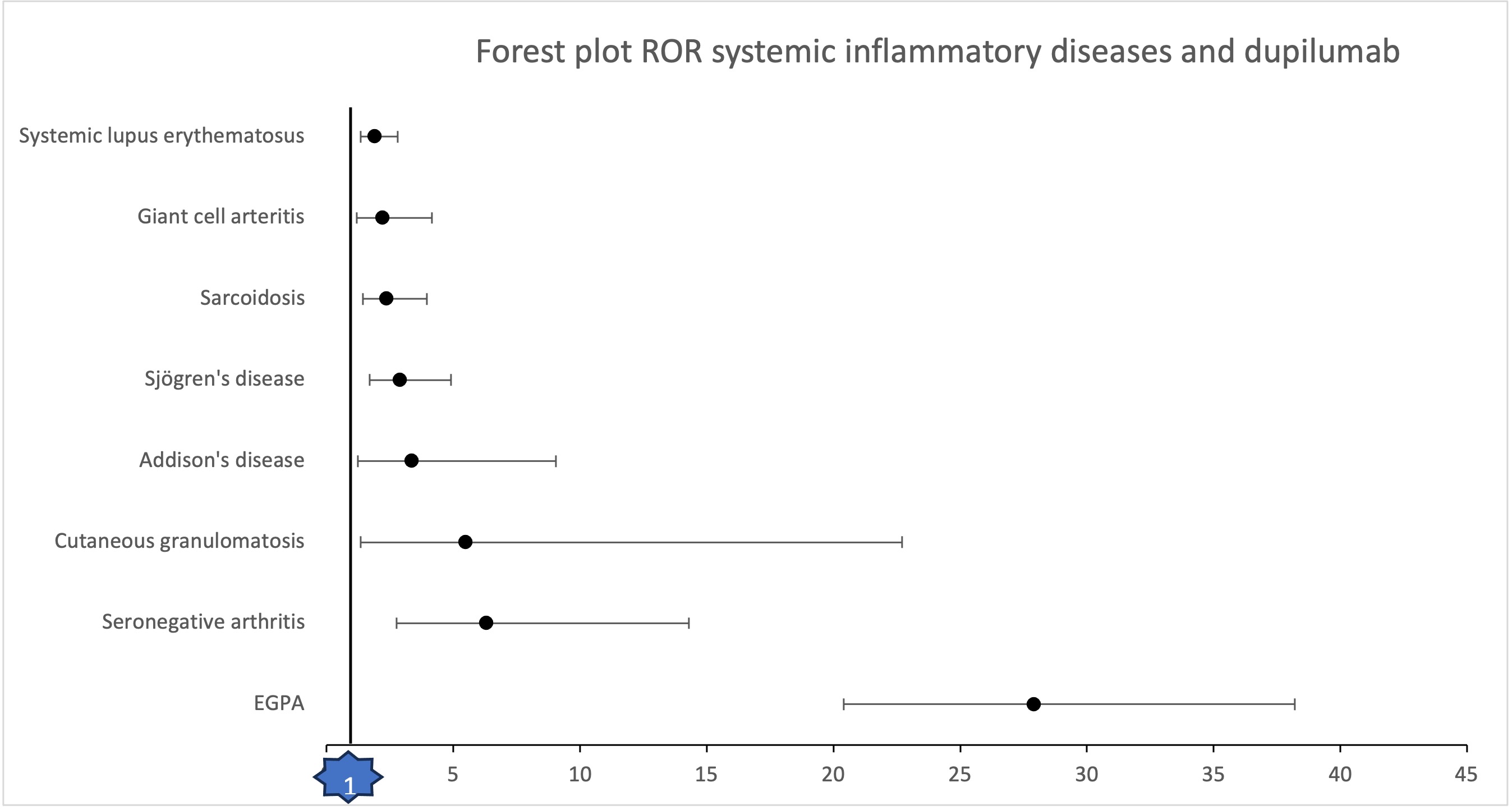
Analysis of the Vigibase revealed a pharmacovigilance signal for the following diseases: eosinophilic granulomatosis with polyangiitis (ROR 27.9; 95%CI 20.4-38.2), seronegative arthritis (ROR 6.30; 95%CI 2.76-14. 3), cutaneous granulomatosis (ROR 5.49; 95%CI 1.33-22.7), Addison’s disease (ROR 3.35; 95%CI 1.24-9.05), Sjögren’s syndrome (ROR 2.88; 95%CI 1.70-4. 9), sarcoidosis (ROR 2.36; 95%CI 1.42-3.95), giant cell arteritis (ROR 2.2; 95%CI 1.19-4.16) and systemic lupus erythematosus (ROR 1.9; 95%CI 1.33-2.8). (Image 1)
Conclusion: Dupilumab appears to be associated with de novo or relapse of systemic inflammatory diseases. These include eosinophilic granulomatosis with polyangiitis, seronegative arthritis, cutaneous granulomatosis, sarcoidosis, giant cell arteritis, Addison’s disease, Sjögren’s syndrome and systemic lupus erythematosus. These data will allow us to improve the monitoring and follow-up of patients receiving dupilumab.
To cite this abstract in AMA style:
Tisseau des Escotais J, Taille C, Merindol J, Groh M, SMETS P, Boudjemaa A, Audemard A, Belfeki N, Bonniaud P, Comarmond C, DELACROIX I, Descours C, Grange L, Legendre P, Martis N, Moulinet T, Walls B, Chouchana L, Terrier B. Induction of Systemic Inflammatory Diseases with Dupilumab Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/induction-of-systemic-inflammatory-diseases-with-dupilumab-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/induction-of-systemic-inflammatory-diseases-with-dupilumab-therapy/