Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Ixekizumab (IXE), an IL-17A antagonist, is effective in patients with radiographic axial spondyloarthritis (rad-axSpA). Assessment in SpondyloArthritis International Society (ASAS) 40 response – the primary study endpoint – was achieved at week (wk) 16 by 48% of those treated with 80mg subcutaneous IXE every 4 wks (Q4W) in the phase 3 COAST V trial (NCT 02696785) 1. Until now, no information has been available on the efficacy of IXE on the components of the ASAS40 composite endpoint. Our objective is to describe which individual components of ASAS40 drive the achievement of efficacy response.
Methods: This exploratory post-hoc analysis was based on COAST V data. Patients enrolled in COAST V met ASAS criteria for rad-axSpA and were biological disease-modifying antirheumatic drug (bDMARD)-naïve. Patients were assigned 1:1:1:1 to subcutaneous placebo (PBO), IXE Q4W, IXE Q2W or 40 mg adalimumab (ADA). Only data for approved doses are shown.
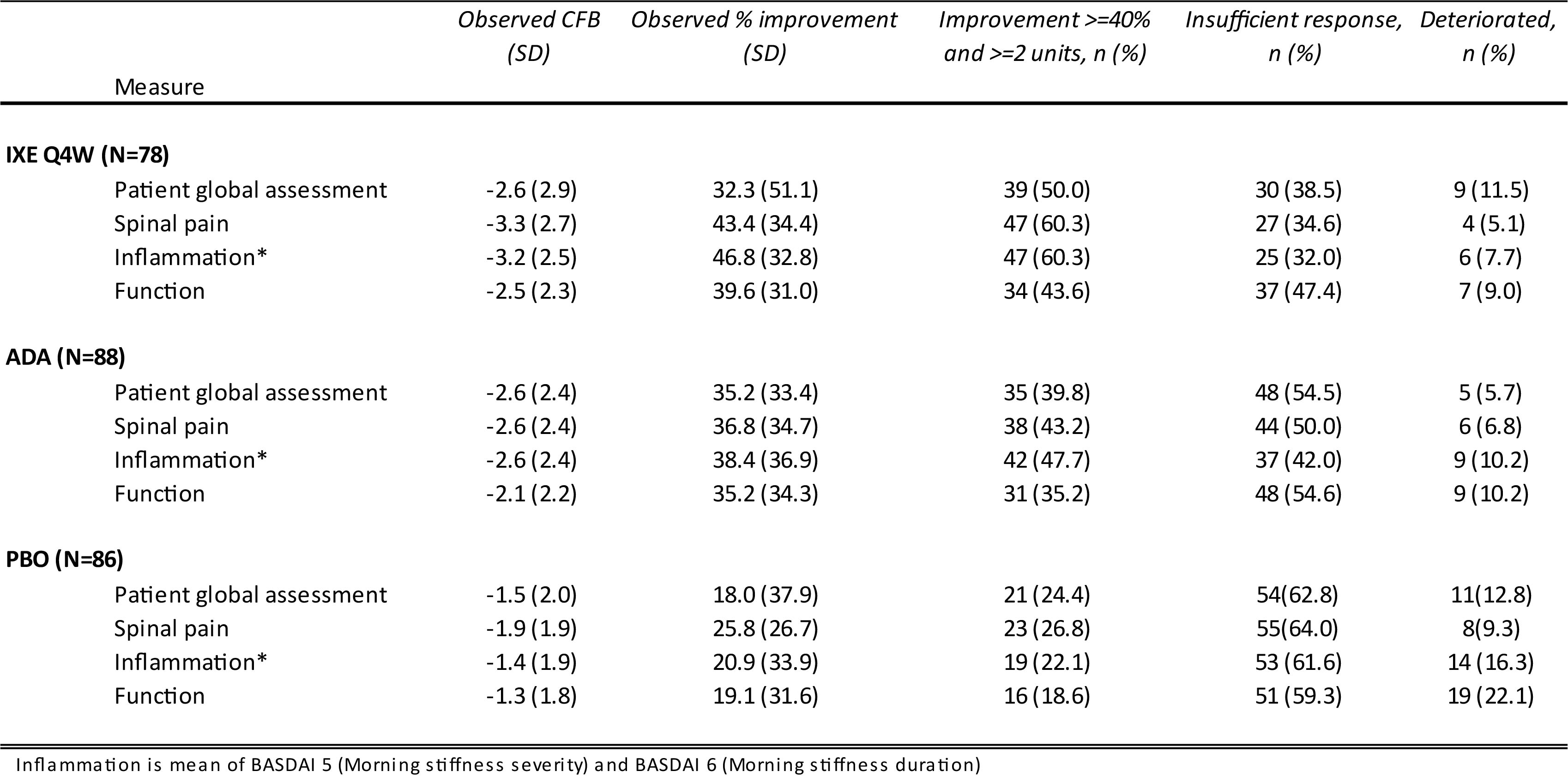
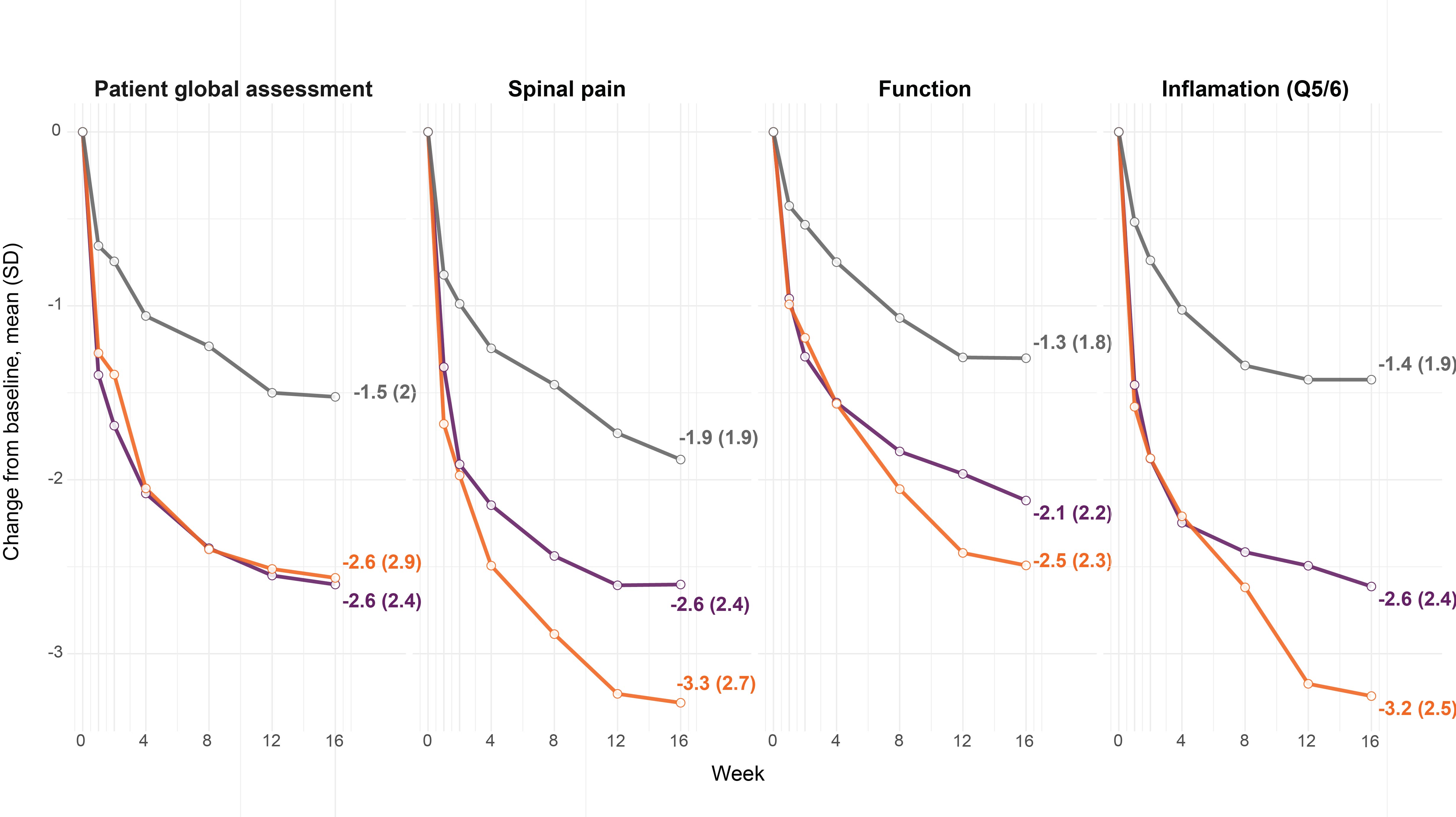
To reach ASAS40 response, patients must have an improvement of at least 40% and at least 2 units for at least 3 of 4 individual components which define response (patient global assessment of disease activity, spinal pain, inflammation (defined as the mean of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) questions 5 and 6), and function (Bath Ankylosing Spondylitis Functional Index – BASFI)), without worsening in the remaining component. We describe the percentage of patients who achieved this change, had an insufficient response, or deteriorated in each component out to wk 16 for IXE Q4W, ADA and PBO. The time course of the change from baseline in individual components of the ASAS response is depicted descriptively per treatment arm by use of the mean and standard deviation. Observed data have been utilised.
Results: IXE Q4W response at 16 wks was driven by all 4 individual components of the ASAS40 with the largest improvements for patients treated with IXE Q4W seen in inflammation and spinal pain (Fig. 1).
At wk 16, at least 50% of all patients treated with IXE Q4W achieved response on spinal pain (60.3%), inflammation (60.3%) and patient global assessment (50%), with 43.6% of patients meeting the response criteria for function (Table 1). The corresponding results for ADA were 43.2%, 47.7% 39.8%, and 35.2%.
Conclusion: Our findings show that meeting ASAS40 response criterion for an individual component at 16 wks by patients treated with IXE Q4W was broadly similar between individual components. However, a clinically relevant improvement was more frequently observed for the spinal pain and inflammation components.
1). Dougados, M., et al. (2020). Ann Rheum Dis 79(2): 176-185.
To cite this abstract in AMA style:
Poddubnyy D, Attar S, Nissen M, Fillipi E, Russ H, Erdogan A, Schymura Y, Liu-Leage S, Collantes-Estevez E, Ciccia F. Individual Components Contributing to the Achievement of Assessment in SpondyloArthritis International Society 40 Response in Biologic Naïve Patients with Radiographic Axial Spondyloarthritis: Results from the COAST V Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/individual-components-contributing-to-the-achievement-of-assessment-in-spondyloarthritis-international-society-40-response-in-biologic-naive-patients-with-radiographic-axial-spondyloarthritis-results/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/individual-components-contributing-to-the-achievement-of-assessment-in-spondyloarthritis-international-society-40-response-in-biologic-naive-patients-with-radiographic-axial-spondyloarthritis-results/