Session Information
Date: Tuesday, October 28, 2025
Title: (2547–2566) ARP Posters I
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Underrepresented Systemic Lupus Erythematosus (SLE) patients are greatly underrepresented in SLE clinical trials. We aimed to increase clinical trial awareness with various interventions in our community practice with a small sample of patients.
Methods: The primary investigator’s underrepresented SLE patients meeting ACR classification for the disease aged 18 and over were offered an electronic survey to assess their knowledge of clinical trials. They were given material about clinical trials and information from the Lupus Foundation and encouraged to contact them for support groups, etc. At year two, surveys were given to the same patients to see if they have increased knowledge of clinical trials in SLE, or to see if any of them have joined a clinical trial in the study period.
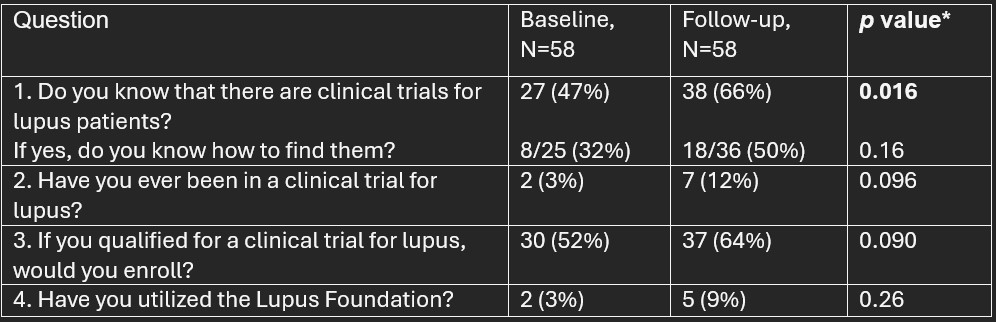
Results: At the end of year one, 86 patients filled out the demographics portion of the survey. Of these, 81% (n=70) % were Black, 14 % (n=12) were Hispanic/Latinx, 2% (n=2) were White, with 1% (n=1) Asian and 1% (n=1) not choose not to report race. Two (2%) of those 86 did not answer the questions regarding resources and could not be included; of those 84, 58 (69%) completed a follow-up questionnaire. One had follow-up data but no baseline data. The 58 who provided data at both visits comprise the sample for analysis, as the goal was to compare baseline and follow-up responses to the same questions. Significant change was observed in the rate of awareness from baseline visit to the follow-up visit. Sixteen additional participants reported being aware of clinical trials at follow-up compared to baseline (while 5 changed from being aware at baseline to not at follow-up). This change is significant, p=0.016. As noted in the table, other areas (such as willingness to participate in a clinical trial if qualified to do so) also observed improved rates at follow-up, but no other changes were significant at the pre-established value of p< 0.05.
Conclusion: SLE is a serious chronic illness and clinical trials are necessary to get more drugs approved for treatment. Underrepresented populations are most severely affected, and yet there are barriers to clinical trial interest and entry. Our data showed that our intervention did increase clinical trial awareness in our small sample of patients. There was a trend for patients to say they would be willing to participate in a clinical trial if qualified to do so, but it did not become clinically significant. This could have been due to a small sample size. We do believe education is key to increasing underrepresented patients in SLE clinical trials.
To cite this abstract in AMA style:
Ranken L, Osborne K, Fairman R, Russell G, Ohl S, Kearney-Bryan J. Increasing Clinical Trial Awareness in Sample of Underrepresented Systemic Lupus Erythematosus Patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/increasing-clinical-trial-awareness-in-sample-of-underrepresented-systemic-lupus-erythematosus-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-clinical-trial-awareness-in-sample-of-underrepresented-systemic-lupus-erythematosus-patients/