Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Biological drugs have revolutionized the treatment of rheumatic diseases. However, increasing the use of biologics over the past few years has directly contributed towards higher prescription drug spending in the United States. It is estimated that low-cost biosimilars will have a considerable impact by reducing prescription drugs spending, lowering the US healthcare costs, and increasing access to care. Although the infusion clinics at our large academic center have been using the biosimilars for Infliximab and Rituximab since 2018 and 2020 respectively, the initial uptake was slow.
Methods: To facilitate their use, biosimilars were made available to order within our electronic heath record (EHR). They were eventually made the default orders, replacing the originator drug orders within the EHR. We measured the effect of the EHR changes on the use of biosimilars for rheumatic diseases since their introduction into our institution. We used aggregated, de-identified data of rheumatology patients who received Infliximab or Rituximab in our infusion clinics between August 2018 through March 2022. The percentage of biosimilars use was calculated using Microsoft Excel.
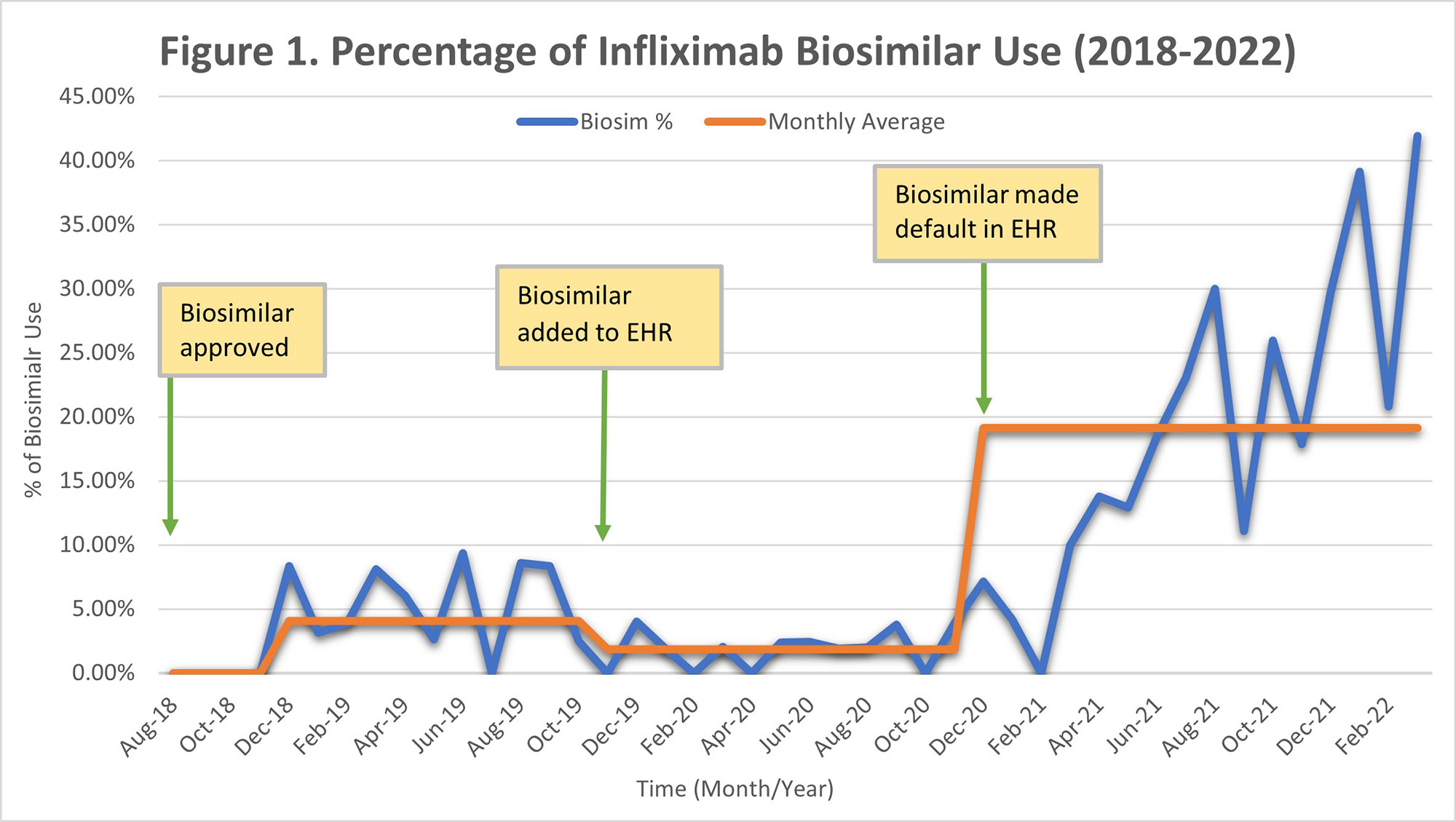
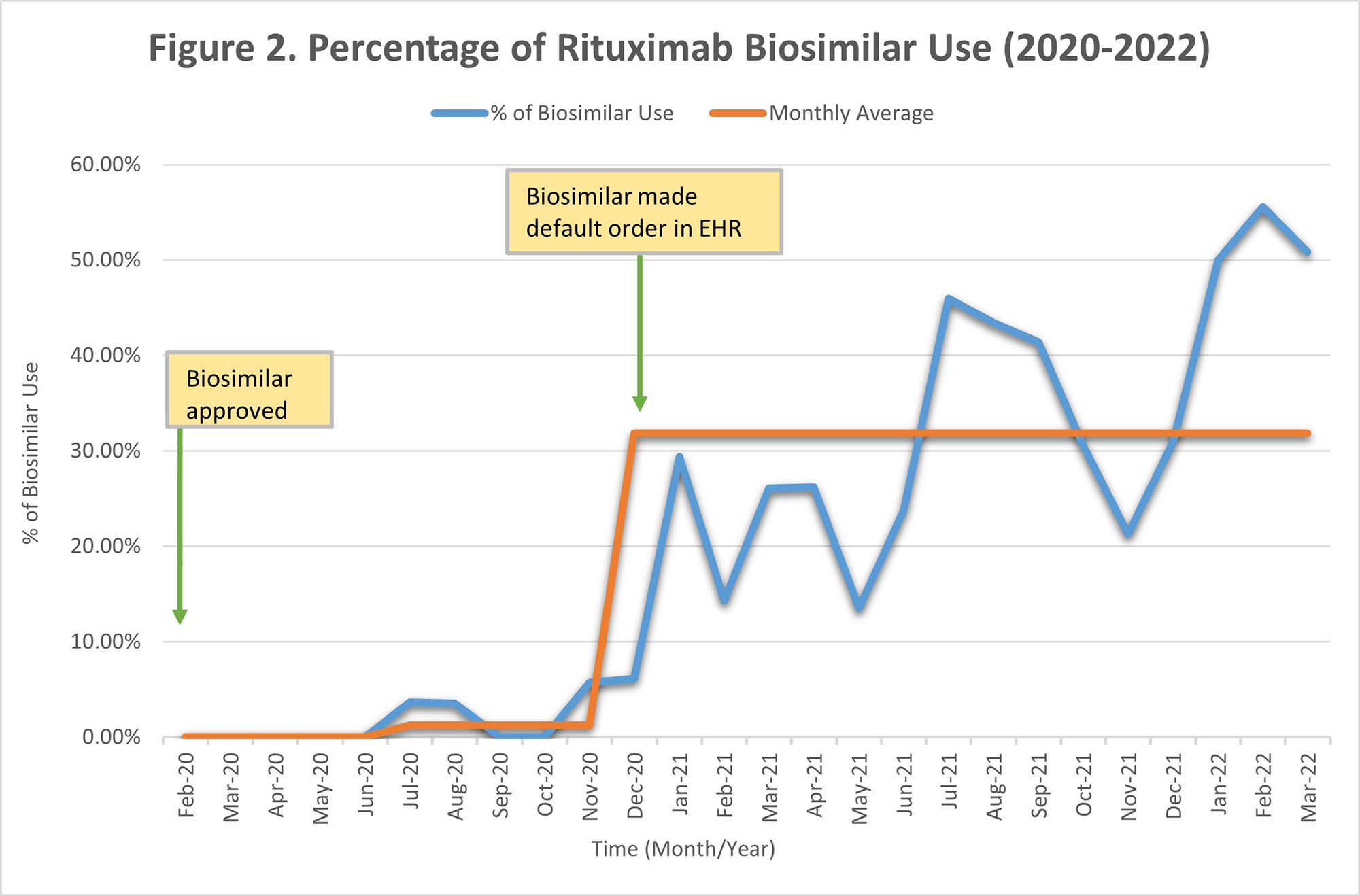
Results: Sixteen rheumatology providers placed a total of 1270 originator infliximab orders and 129 biosimilar orders between August 2018 and March 2022. There were 1295 total rituximab originator orders and 208 biosimilar orders between February 2020 and March 2022. After the approval and introduction of infliximab biosimilar in August 2018, the average monthly percentage use was 4.06%, which changed to 1.87% after it was added as an option in the EHR order set in November 2019. The monthly average use increased to 19.13% after the biosimilar was made the default order, replacing the originator drug in December 2020 (Figure 1). The average monthly percentage use for rituximab biosimilar gradually increased to 1.30% after it was approved and added as an optional order in the EHR in February 2020. This average increased to 31.84% after the biosimilar was made the default order, replacing the originator drug in December 2020 (Figure 2).
Conclusion: It is feasible to increase the use of biosimilars in an academic setting. In our institution, a significant increase in uptake was not seen until the biosimilar drug was made the default EHR order, replacing the originator drug. With an estimated cost that can be up to 40-50% less than the originator drugs, we estimate that the continued increase in biosimilars uptake will lead to significant savings. We plan to compare the biosimilar drugs uptake within rheumatology with other specialties and continue to develop interventions to increase biosimilar use in the future.
To cite this abstract in AMA style:
Eseddi J, Carmichael D, Wishin S, Bajaj P. Increasing Biosimilar Uptake in the Rheumatology Clinics Within a Large Academic Medical Center [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/increasing-biosimilar-uptake-in-the-rheumatology-clinics-within-a-large-academic-medical-center/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-biosimilar-uptake-in-the-rheumatology-clinics-within-a-large-academic-medical-center/