Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Aside from the disease itself, infections represent the major cause of morbidity and mortality in systemic lupus erythematosus (SLE) patients. Although the daily usage of immunosuppressive drugs is considered to be responsible for the increased rates of infection, inherent defects in immune system should not be overlooked.
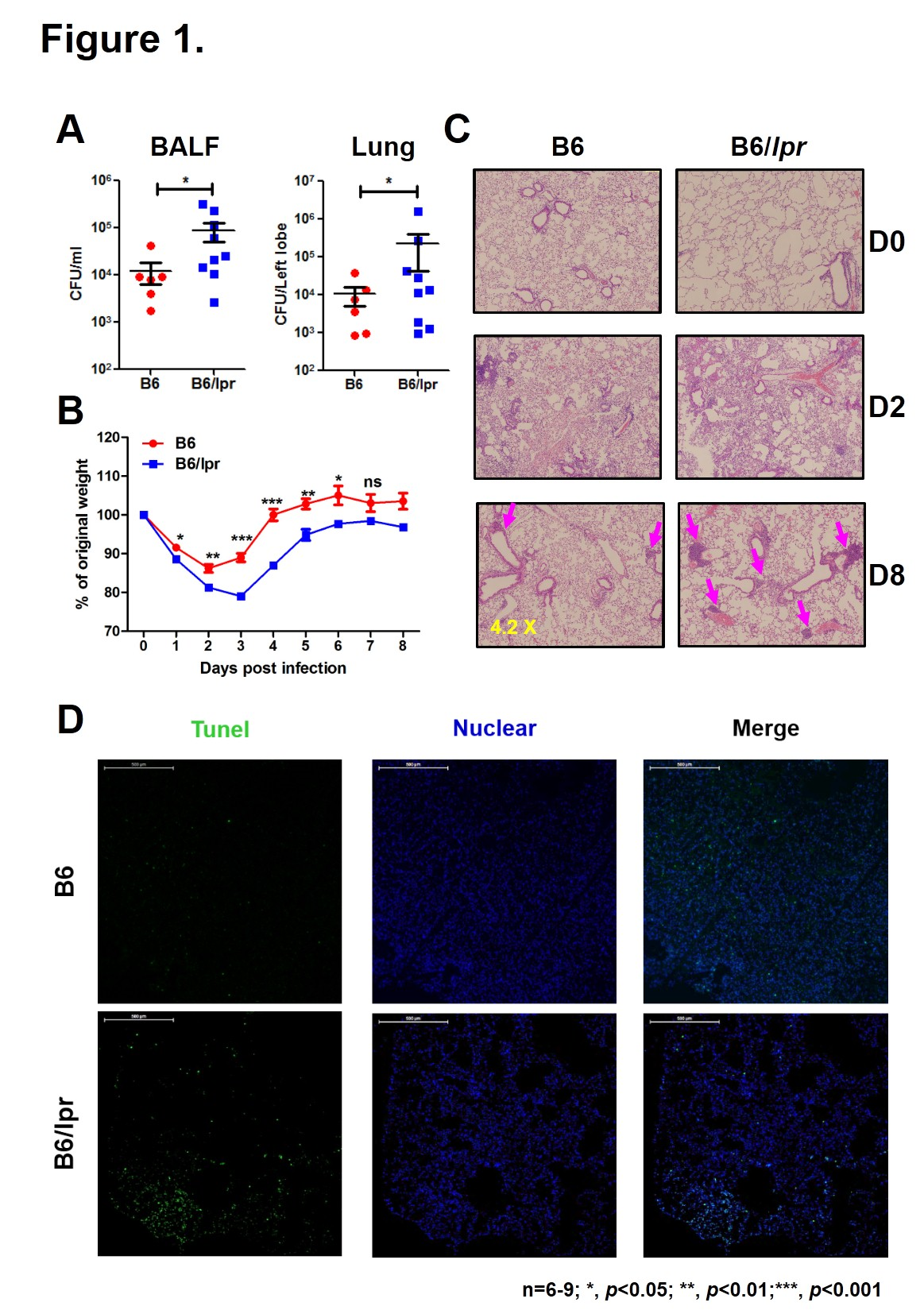
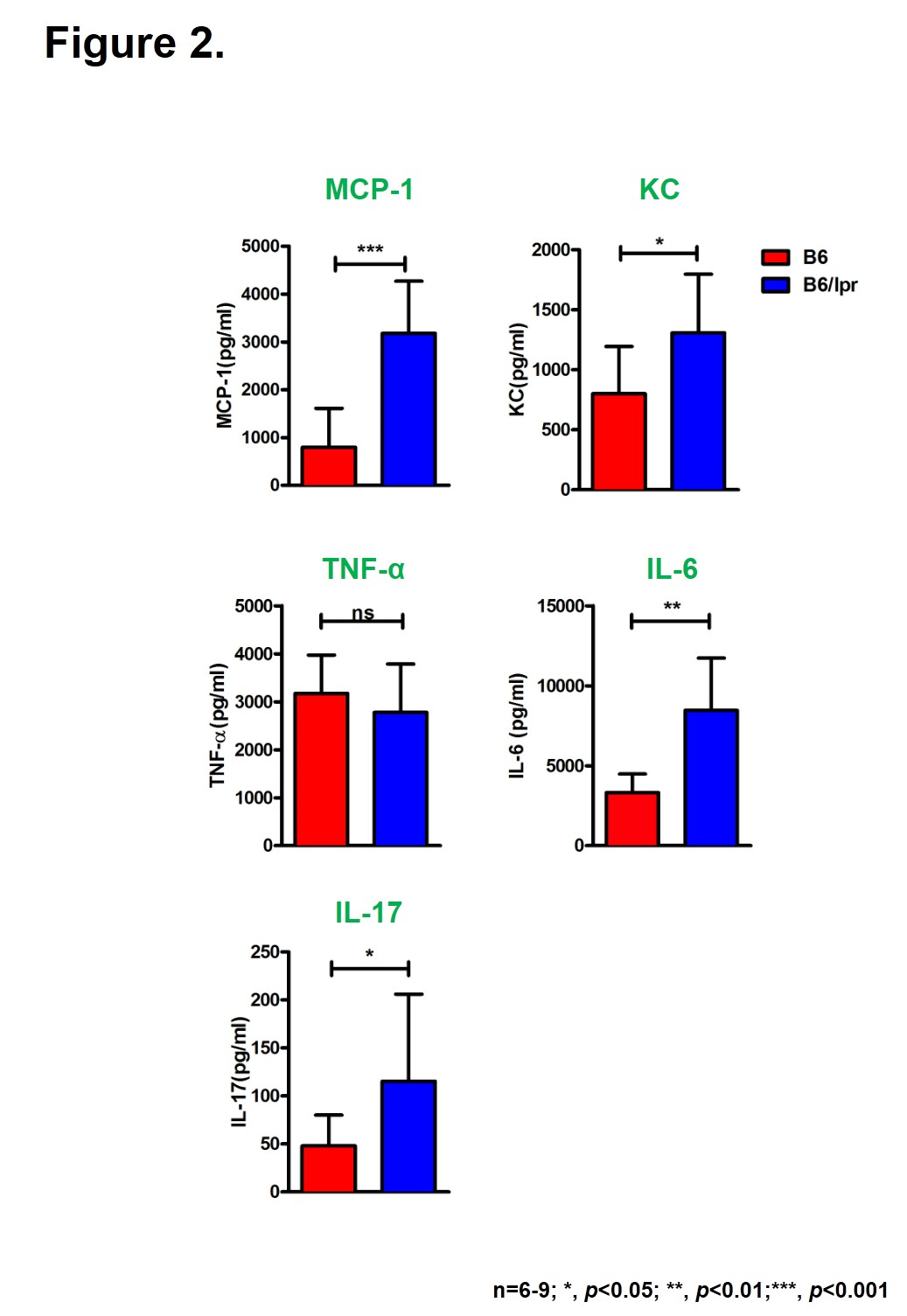
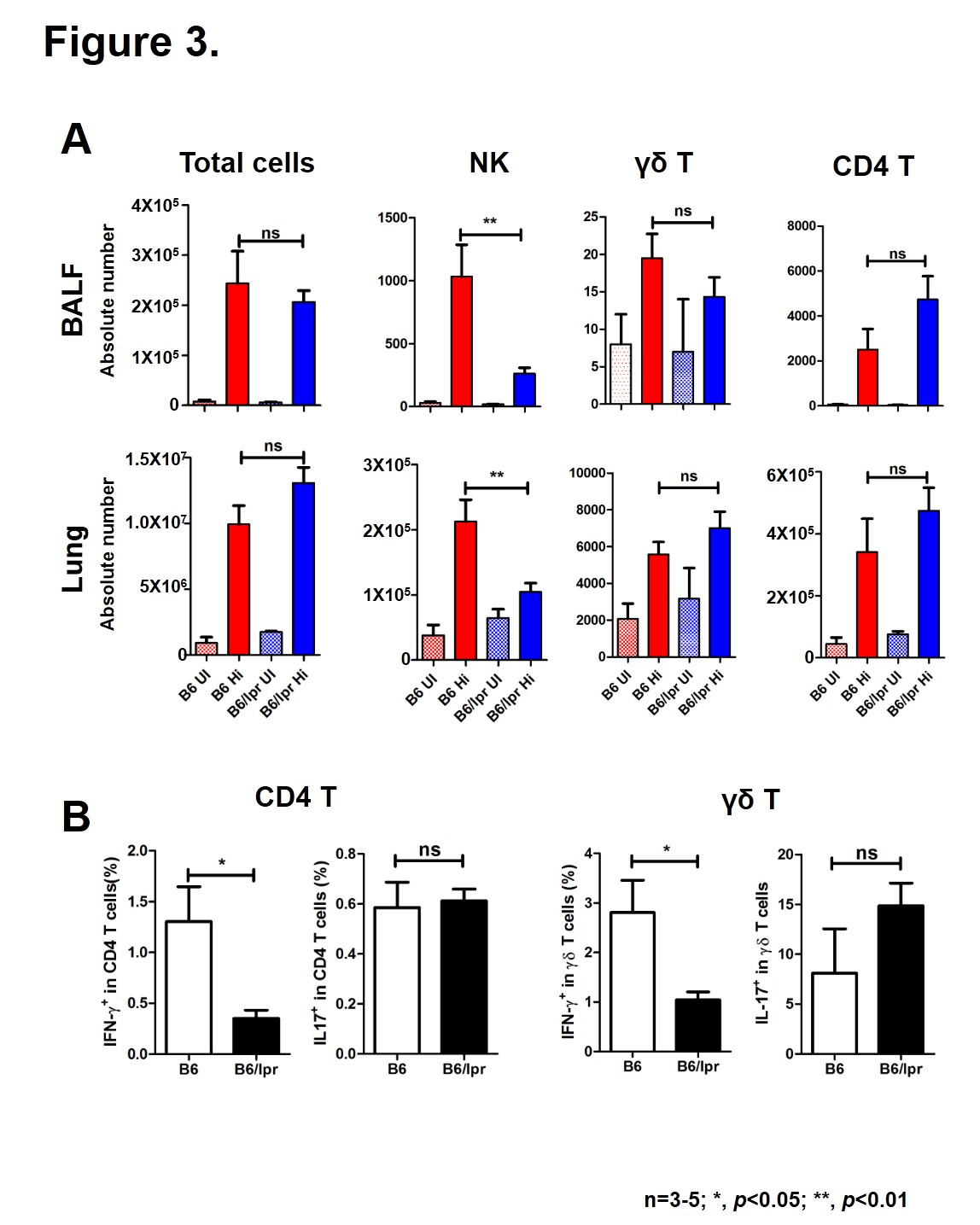
Methods: To better understand the proclivity of SLE patients to suffer infections, we infected lupus-prone mice B6/lpr with 1X10^8 CFU of Haemophilus influenzae (Hi) intranasally and monitored bacterial clearance, body weight change and lung pathology after infection. Apoptosis of lung cells was analyzed by TUNEL assay. Immune cells recruited by infection were determined by flow cytometry. Cytokines in the bronchoalveolar lavage fluid (BALF) were measured by ELISA.
Results: Although both wild-type (WT) and B6/lpr mice survived after pulmonary Hi infection, a delay of bacterial clearance and inflammatory resolution was observed in B6/lpr mice(Fig1A-C). Besides, tissue damage was more severe in B6/lpr mice, as more apoptotic cells appeared in the lung on D2 after infection (Fig1D). When looked at the cytokine production, we found that cells from lupus-prone lungs produced much more proinflammatory cytokines IL-6, IL-17 and chemokines MCP-1 and KC. However, TNF-¦Á is comparable between the two groups (Fig2). NK, ¦æÄ T and CD4 T cells are very important in control of bacterial infection. Here, we showed that compared with WT controls, in response to infection fewer NK cells were detected in B6/lpr lungs. The numbers of ¦æÄ T and CD4 T cells in the lung were not different, but their ability to secrete IFN-¦Ã was significant lower in B6/lpr mice(Fig3).
Conclusion: The increased susceptibility of SLE-prone mice to pulmonary Haemophilus influenzae infection may due to the elevated inflammatory responses and the deficiency of immune cells.
To cite this abstract in AMA style:
Li W, Sun L. Increased Susceptibility of SLE-Prone Mice to Pulmonary Haemophilus Influenzae Infection Was Attributed to Dysfunctions of Innate Immune Responses [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/increased-susceptibility-of-sle-prone-mice-to-pulmonary-haemophilus-influenzae-infection-was-attributed-to-dysfunctions-of-innate-immune-responses/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-susceptibility-of-sle-prone-mice-to-pulmonary-haemophilus-influenzae-infection-was-attributed-to-dysfunctions-of-innate-immune-responses/