Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The interferon (IFN) signature in SLE is well established, distinguishing lupus patients from healthy controls. Additionally, within lupus patients, higher levels of IFN-responsive gene expression associate with higher disease activity, elevated autoantibodies, greater number of SLE criteria, and higher damage indices. Despite these associations in SLE patients, an individual’s IFN signature lacks responsiveness to acute changes in disease activity in longitudinal analyses. This stability is more reflective of a continuous trait, likely the result of known interferon pathway genetic associations. Elevated levels of IFN have been noted in lupus nephritis, which occurs in approximately half of SLE patients and is a major cause of morbidity and early mortality. Delay in diagnosis of lupus nephritis results in prolongation of renal inflammation, and often irreversible kidney damage. The ability to predict patients at greater risk of lupus nephritis may improve surveillance, reduce time to diagnosis and treatment, and potentially result in improved outcomes in lupus nephritis. We evaluated the prognostic significance of an individual having an elevated IFN-signature trait and progression to lupus nephritis.
Methods: The study included 201 lupus patients, all meeting both the ACR 1997 and SLICC 2012 classification criteria for SLE from a single institution. The open cohort had a median follow-up time of 14.14 years [IQR 10.96, 19.83]. Stored whole blood RNA (PAXgene) samples from the earliest longitudinal timepoint on these 201 patients were assessed for interferon signatures using an IFN-responsive four gene expression assay (Autoimmune Profile Assay, DxTerity Diagnostics) to define an individual’s overall IFN-signature trait. Lupus nephritis status was defined by the date of attainment of the renal component of the SLICC SLE classification criteria. Cox proportional hazards modeling was utilized to determine the contribution of IFN signature trait, age at SLE diagnosis, gender, and race to the development of lupus nephritis.
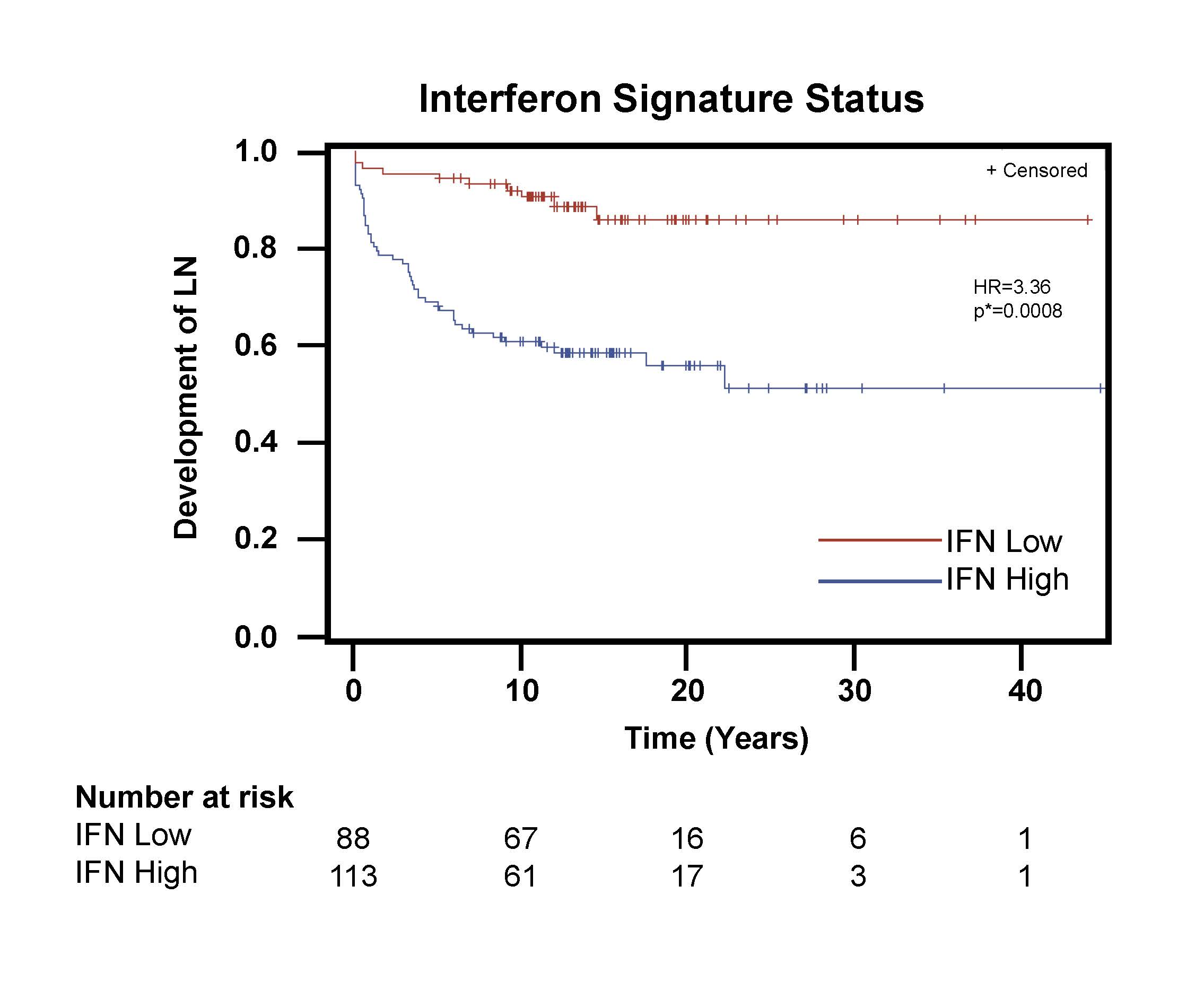
Results: The cohort of 201 SLE patients included 58 patients who developed lupus nephritis and 113 with a high IFN signature trait. Characteristics of the complete group, as well as IFN-signature subgroups, are displayed in Table 1. High IFN signature trait was an independent predictor for earlier time to development of nephritis (Hazard Ratio 3.36, p=0.0008) after adjusting for age at SLE diagnosis, gender, and race (Figure 1). In our Cox proportional hazards model, younger age at diagnosis of SLE, male gender, and non-white race were also associated with higher likelihood of nephritis development. Racial subgroup analyses found that high IFN signature remained a significant predictor of earlier nephritis when evaluating either non-white patients (Hazard Ratio 3.41, p=0.021) or white patients (Hazard Ratio 2.97, p=0.031), adjusting for age at SLE diagnosis and gender.
Conclusion: Assessment of an individual’s interferon signature phenotypic trait at the time of SLE diagnosis may be a useful tool to determine risk for development of lupus nephritis.
To cite this abstract in AMA style:
Arriens C, Raja Q, Husain S, George B, Abedi M, Jacobs A, Guyon T, Wijesuriya H, Aberle T, Thanou A, Kamp S, Macwana S, Chakravarty E, Merrill J, James J, Terbrueggen R, Guthridge J. Increased Risk of Progression to Lupus Nephritis for Lupus Patients with Elevated Interferon Signature [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/increased-risk-of-progression-to-lupus-nephritis-for-lupus-patients-with-elevated-interferon-signature/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-risk-of-progression-to-lupus-nephritis-for-lupus-patients-with-elevated-interferon-signature/