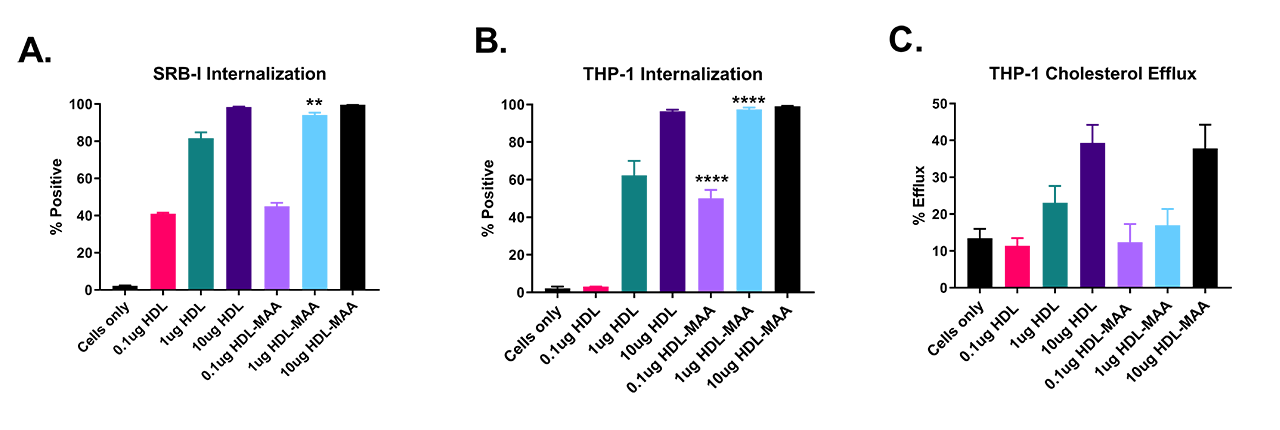Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatoid arthritis (RA) are approximately two-fold more likely to develop cardiovascular disease (CVD). Prior reports have suggested that “dysfunctional” HDL may explain at least part of this enhanced risk profile. It has been suggested that post-translational modifications of HDL, including those characteristic of RA, may attenuate relevant protective properties of this lipoprotein. Specifically, studies have suggested that the irreversible post-translational modification of proteins with malondialdehyde-acetaldehyde (MAA) that are prevalent in RA synovium are also present in atherosclerotic plaque. Moreover, antibodies to MAA correlate with the development and progression of both RA and CVD. Therefore, the purpose of this study was to examine the biologic effects resulting from the exposure of MAA-modified HDL to human macrophages that have been implicated in CVD pathogenesis.
Methods: HDL was incubated with 2mM malondialdehye and 1mM acetaldehyde for 3 days to form the MAA adduct. PMA activated human THP-1 macrophages were subjected to 0.1, 1, 10 μg/ml of native or modified HDL in viability assays for 4 hours and ligand internalization studies for 90 minutes. Internalization studies were also performed using Chinese Hamster Ovary (CHO) cells transfected with the B-I scavenger receptor (SRB-I) shown in prior studies to mediate cellular uptake of MAA-modified protein. Measurement of modified HDL function was tested using a fluorescent labeled cholesterol efflux assay using THP-1 cells.
Results: MAA modification of HDL was evident after 72 hours by fluorescence of the dihydropyridine ring at 398nm. THP-1 cell viability remained >95% after 4 hours of exposure to HDL-MAA. HDL-MAA was internalized at increasing concentrations compared to native HDL in both CHO cells transfected with the SRB-I receptor (p< 0.01) (A) and THP-1 cells (p< 0.0001) (B). However, cholesterol efflux (C) was not significantly different between native HDL and HDL-MAA, indicating that cholesterol binding is not MAA-dependent.
Conclusion: Post-translational modification of HDL may contribute to the development and progression of RA and CVD comorbidity. Increased internalization of HDL-MAA in the absence of meaningful effects on cholesterol efflux suggests that MAA modification does not alter the physiological rate of reverse cholesterol transport. However, the preferential accumulation of HDL in THP-1 cells following MAA modification suggests that this post-translational modification (demonstrated in RA to result from inflammation and increased oxidative stress) could impact the development and progression of comorbid CVD.
To cite this abstract in AMA style:
Real K, Duryee M, Opperman P, Ryan E, Duryee L, O'Dell J, Mikuls T, Anderson D, Thiele G. Increased Accumulation of Malondialdehyde-Acetaldehyde Modified HDL in Macrophage Without Decreased Cholesterol Efflux [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/increased-accumulation-of-malondialdehyde-acetaldehyde-modified-hdl-in-macrophage-without-decreased-cholesterol-efflux/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-accumulation-of-malondialdehyde-acetaldehyde-modified-hdl-in-macrophage-without-decreased-cholesterol-efflux/