Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Pneumocystis jiroveci pneumonia (PJP) is an opportunistic infection that may affect patients with ANCA vasculitis (AAV). Current guidelines conditionally recommend PJP prophylaxis for patients with AAV who are being treated with rituximab or cyclophosphamide, but the quality of evidence was rated as “low.” The objective of this study was to estimate the incidence of PJP infections among new users of rituximab or cyclophosphamide who had AAV and to evaluate the risk of toxicities commonly associated with PJP prophylaxis.
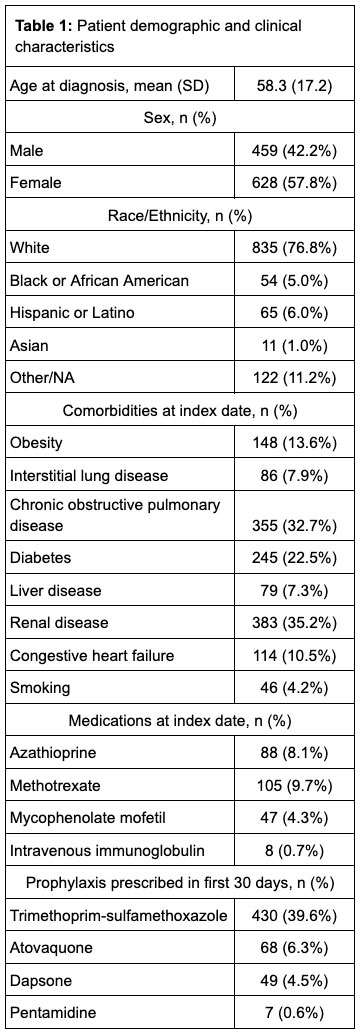
Methods: A retrospective cohort study was conducted using data from the TriNetX (Cambridge, MA, USA) electronic health records database, which includes records from 56 United States health organizations. This study used records from 1993-2001. Patients were included if they met the following criteria: (1) ≥ 2 ICD-9-CM/ICD-10 codes for AAV (446.0, 446.4, M30.0, M31.30, M30.1, M31.31, M31.7) separated by at least 30 days; (2) ≥ 1 administration of rituximab or cyclophosphamide (intravenous or oral); and (3) ≥1 prescription for prednisone within 30 days of the first prescription for rituximab or cyclophosphamide. Patients were followed from the index date (first administration of rituximab or cyclophosphamide on or after the date of the first AAV code) until PJP, death, or 6 months after the index date. PJP was defined by any hospitalization with primary or secondary diagnosis by ICD9/ICD10 code, any PJP code followed by treatment, or a positive PJP test. AAV patients who received PJP prophylaxis were propensity score matched (1:1) to patients who did not by the following variables: age, gender, race/ethnicity, region, admissions in the prior year, and comorbidities including obesity, smoking status, congestive heart failure, chronic obstructive pulmonary disease, diabetes, liver disease, and cardiovascular disease. Incident rate ratios (IRR) for PJP and for adverse events commonly associated with PJP prophylaxis were estimated.
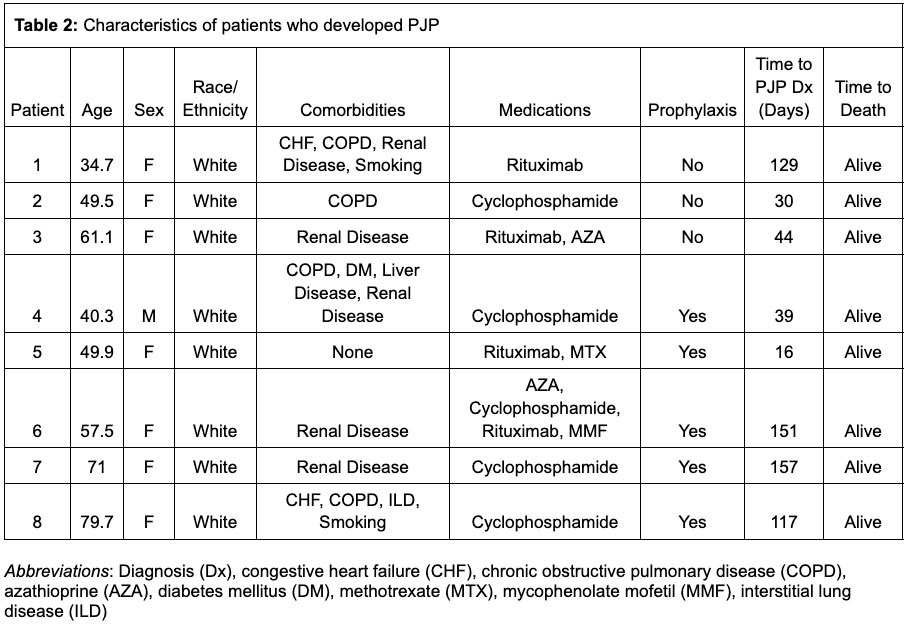
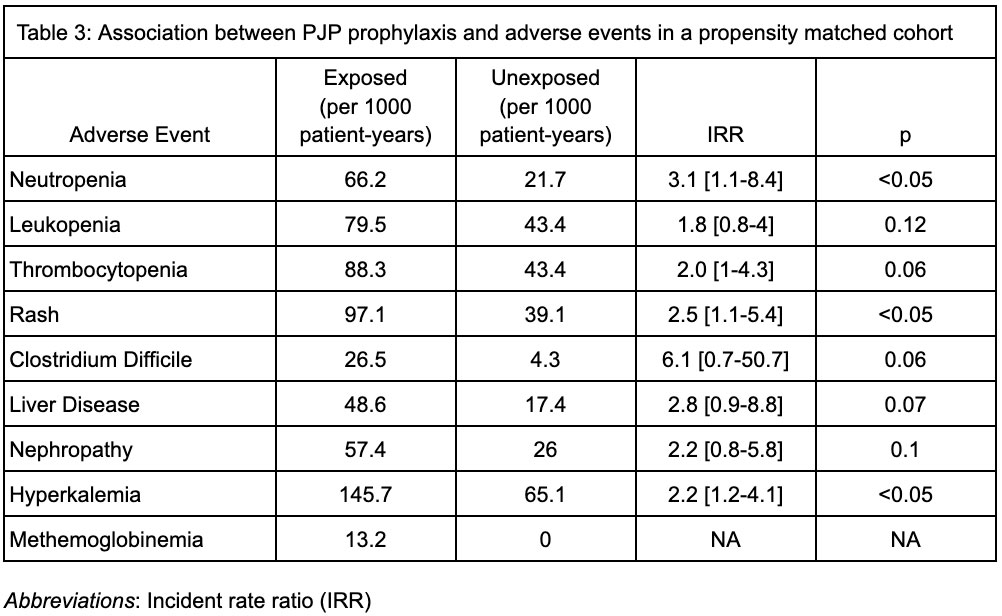
Results: We identified 1087 cases of AAV, who were followed for an average of 169 (SD 35.6) days. The mean weighted Charlson Comorbidity Index (CCl) was 1.6 (SD 1.9). 484 patients (45%) were treated with cyclophosphamide and 697 patients (64%) were treated with rituximab. Prophylactic medications for PJP prescribed within the first 30 days included trimethoprim-sulfamethoxazole (39.6%), atovaquone (6.3%), dapsone (4.5%) and pentamidine (0.6%). Over 504 person years of follow up, 8 cases of PJP were identified (incidence rate 15.9 cases per 1000 patient-years), 5 of whom received prophylaxis; no deaths or hospitalizations for PJP were observed. After propensity score matching (n = 988), there was no significant association between PJP prophylaxis and developing PJP (IRR 1.7, 95% confidence interval (CI) 0.4-7.1), but neutropenia (IRR 3.1, CI 1.1-8.4), rash (IRR 2.5, CI 1.1-5.4) and hyperkalemia (IRR 2.2, CI 1.2-4.1) were more common in the group receiving prophylaxis.
Conclusion: PJP occurs in patients with AAV who undergo treatment with cyclophosphamide or rituximab, but prophylaxis against PJP is associated with increased incidence of medication-related adverse events. Future studies should define individual risk-benefit approaches for patients with AAV.
To cite this abstract in AMA style:
Nettleton E, Sattui S, Wallace Z, Putman M. Incidence of Pneumocystis Jiroveci Pneumonia in Patients with ANCA Vasculitis Initiating Therapy with Rituximab or Cyclophosphamide [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/incidence-of-pneumocystis-jiroveci-pneumonia-in-patients-with-anca-vasculitis-initiating-therapy-with-rituximab-or-cyclophosphamide/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-pneumocystis-jiroveci-pneumonia-in-patients-with-anca-vasculitis-initiating-therapy-with-rituximab-or-cyclophosphamide/