Session Information
Date: Monday, November 9, 2020
Title: RA – Treatments II: Potential Harms & Adverse Events (1998–2002)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Upadacitinib (UPA) is an oral JAK inhibitor approved for the treatment of rheumatoid arthritis (RA). The background rate of herpes zoster (HZ) in patients (pts) with RA is around 0.98/100 person years (PY)1. Pts with RA receiving JAK inhibitors have been reported to have an increased risk of HZ. We evaluate the incidence and risk factors for HZ in pts with RA receiving UPA relative to active comparators in the Phase 3 clinical trial program.
Methods: The incidence rate of HZ was determined in pts receiving UPA (as monotherapy [mono] or combination therapy) in five randomized Phase 3 trials (SELECT-EARLY, SELECT-MONOTHERAPY, SELECT-NEXT, SELECT-COMPARE, and SELECT-BEYOND), of which 4 evaluated both the UPA 15 and 30 mg once-daily (QD) doses and 1 trial (SELECT-COMPARE) evaluated only the 15 mg QD dose. Incidence of HZ was also determined in pts receiving adalimumab (ADA) + methotrexate (MTX) in SELECT-COMPARE and MTX mono in SELECT-EARLY. Risk factors for HZ were assessed using univariate and multivariate Cox regression models. Data cut-off was 30 June 2019.
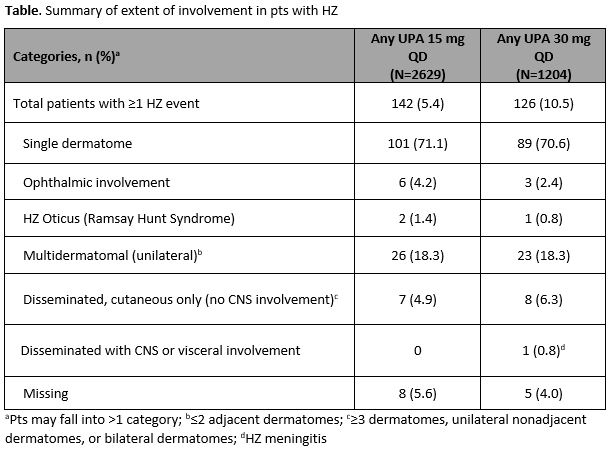
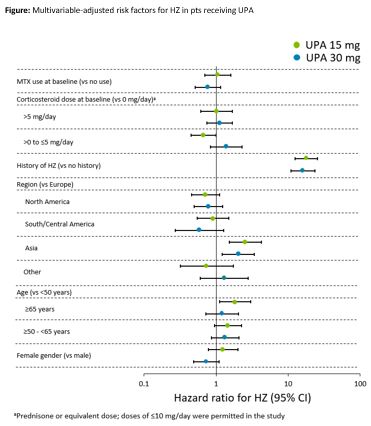
Results: Overall, 2629 pts who received UPA 15 mg QD (4565.8 patient-years [PY]), 1204 pts who received UPA 30 mg QD (2309.7 PY), 579 pts who received ADA + MTX (768.6 PY), and 314 pts who received MTX mono (456.0 PY) were analyzed. Fewer than 5% of pts across the treatment groups reported prior HZ vaccination. HZ (n/100 PY [95% CI]) occurred in 142 pts (3.1 [2.6–3.7]) with UPA 15 mg, 126 pts (5.5 [4.5–6.5]) with UPA 30 mg, 8 pts (1.0 [0.4–2.1]) with ADA + MTX, and 5 pts (1.1 [0.4–2.6]) with MTX mono. Most of the HZ cases (~71%) with UPA (Table) and all cases with ADA + MTX and MTX mono involved a single dermatome. Ophthalmic involvement was seen in 6 (4.2%) and 3 (2.4%) cases in the UPA 15 and 30 mg groups, respectively, and unilateral involvement with multiple dermatomes was seen in 26 (18.3%) and 23 (18.3%) cases. There was a single case of HZ meningitis reported in a Japanese pt on UPA 30 mg. In multivariate analyses, prior history of HZ and Asian region were associated with an increased risk of HZ in both the UPA groups (p≤0.01; Figure). In addition, pts ≥65 years old had increased risk of HZ in the 15 mg group.
Conclusion: HZ events in pts with RA receiving UPA were more common in the 30 mg vs 15 mg group, and in both UPA groups compared with the ADA + MTX and MTX groups.
References:
1. Smitten AL, et al. Arthritis Rheum 2007;57:1431–8
Original abs: Ann Rheum Dis. 2020; 79(S1):331.
To cite this abstract in AMA style:
Winthrop K, Nash P, Yamaoka K, Mysler E, Calabrese L, Khan N, Enejosa J, Song Y, Suboticki J, Curtis J. Incidence and Risk Factors for Herpes Zoster in Rheumatoid Arthritis Patients Receiving Upadacitinib [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/incidence-and-risk-factors-for-herpes-zoster-in-rheumatoid-arthritis-patients-receiving-upadacitinib/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-and-risk-factors-for-herpes-zoster-in-rheumatoid-arthritis-patients-receiving-upadacitinib/