Session Information
Date: Monday, October 22, 2018
Title: Systemic Sclerosis and Related Disorders – Basic Science Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The TbRIIDk-fib transgenic (TG) mouse model of scleroderma carries a fibroblast-specific transforming growth factor β (TGFβ) receptor II mutation resulting in balanced up-regulation of TGFβ signalling. Published data show an excessive pulmonary fibrotic response persists in TG mice in response to intra-tracheal bleomycin administration when compared to unbuffered saline in TG mice or bleomycin in wildtype (WT) mice (1). The pan-peroxisome proliferator-activated receptor (PPAR) agonist lanifibranor (formerly known as IVA337) is currently being tested in a phase II clinical trial in scleroderma and has previously been evaluated in 2 mouse models of scleroderma lung fibrosis (2). In this study, we investigate whether lanifibranor treatment leads to an amelioration of persistent bleomycin-induced lung fibrosis in TbRIIDk-fib mice.
Methods: TG (n=46) and WT mice (n=33) were administered one of two doses of lanifibranor (30 mg/kg or 100 mg/kg) or vehicle administered by daily oral gavage up to 4 weeks. On day 2 bleomycin or unbuffered normal saline (pH 5.3) were administered by oropharyngeal aspiration to trigger lung fibrosis, assessed by histological scoring (Ashcroft score) and biochemical testing of lung homogenates. All procedures were licensed and approved by an animal use ethics committee.
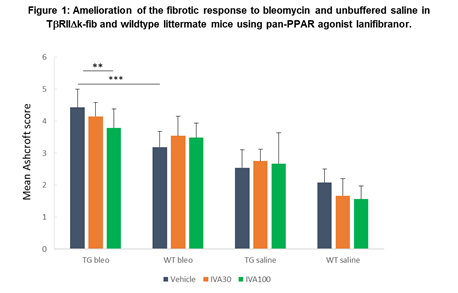
Results: As expected, TG mice demonstrated an exaggerated fibrotic response to oropharyngeal aspiration of bleomycin compared to WT, and to unbuffered saline compared to WT animals (figure 1); demonstrating similar impact when compared with the more invasive intra-tracheal administration published previously.
TG mice treated with higher dose lanifibranor demonstrated significantly greater protection from lung fibrosis than those treated with vehicle or lower dose lanifibranor, for instance: mean Ashcroft score TG-bleo IVA100 3.8 ±0.58; TG-bleo vehicle 4.4±0.58; p<0.01. Treatment with lanifibranor in WT animals was less effective at preventing fibrosis (WT-bleo IVA100 3.5±0.46; WT-bleo vehicle 3.2±0.5; not significant), suggesting that the pro-fibrotic phenotype due to TGF-b upregulation in this model is substantially ameliorated by lanifibranor.
In this study, administration of bleomycin to a transgenic mouse resulted in an Ashcroft score 65% higher compared to saline administration in WT mice. This excessive fibrosis was then reduced by 15% with the use of lanifibranor at 100mg/kg.
Conclusion: Treatment with 100 mg/kg lanifibranor ameliorates lung fibrosis in the TbRIIDk-fib mouse model of scleroderma. This model, which demonstrates severe and persistent fibrosis compared to WT mice, provides mechanistic support for trials of lanifibranor in scleroderma including cases with pulmonary involvement.
References:
1. Hoyles RK et al; Arthritis Rheum. 2008;58:1175-88.
2. Avouac J et al; Ann Rheum Dis. 2017;76:1931-1940.
To cite this abstract in AMA style:
Derrett-Smith EC, Xu S, Abraham D, Lacombe O, Broqua P, Junien JL, Konstantinova I, Denton CP. In Vivo Assessment of Prevention of Lung Fibrosis Using the Pan-PPAR Agonist Lanifibranor in the Tβriiδk-Fib Mouse Model of Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/in-vivo-assessment-of-prevention-of-lung-fibrosis-using-the-pan-ppar-agonist-lanifibranor-in-the-t%ce%b2rii%ce%b4k-fib-mouse-model-of-systemic-sclerosis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-vivo-assessment-of-prevention-of-lung-fibrosis-using-the-pan-ppar-agonist-lanifibranor-in-the-t%ce%b2rii%ce%b4k-fib-mouse-model-of-systemic-sclerosis/