Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: The main treatments for adult and juvenile dermatomyositis (DM/JDM) are immunosuppressant drugs and corticosteroids, which have significant side effects and are not effective in all patients; therefore, identifying new therapeutic strategies for DM/JDM is an important clinical need. Type I Interferons (IFNs) are a group of cytokines including IFN alpha and IFN beta. Type I IFN signalling is detected across myositis subtypes but is particularly strong in DM/JDM. There is increasing evidence that type I IFN signalling in DM/JDM is associated with IFN beta and correlates with disease severity. Therefore, an siRNA targeting the knockdown of expression of the human IFN beta gene IFNB1 is an attractive therapeutic approach in DM/JDM.
Methods: A panel of siRNA targeting IFNB1 was designed with optimisation of GC content and predicted off-target effects, secondary structures, and thermodynamic stability. Fully chemically modified oligonucleotides were synthesised using solid phase synthesis. The oligonucleotides were then purified using reversed-phase HPLC, desalted, and quality control checks performed using LC-MS analysis.
In vitro screening was performed using immortalised myoblasts (Institut de Myologie, Paris). Cells were seeded at 50,000 cells per well of a 24-well plate. In the myoblast screen cells were incubated overnight before treatment. For the myotube screen, cells were grown until 90% confluent and then switched to differentiation media (DMEM with 2% horse serum) for 5-10 days until cells had fused into multinucleated myotubes.
Since healthy cells do not express high levels of IFN beta, expression was induced by transfection with 1ng/mL Poly I:C for 24 hours and then transfected with siRNA for a further 48 hours. Cells were harvested and RNA was isolated for RTqPCR for IFNB1 with HPRT1 or GAPDH as the reference gene. Cell media was collected for IFN beta enzyme linked immunosorbent assay (ELISA) (Abcam ab278127).
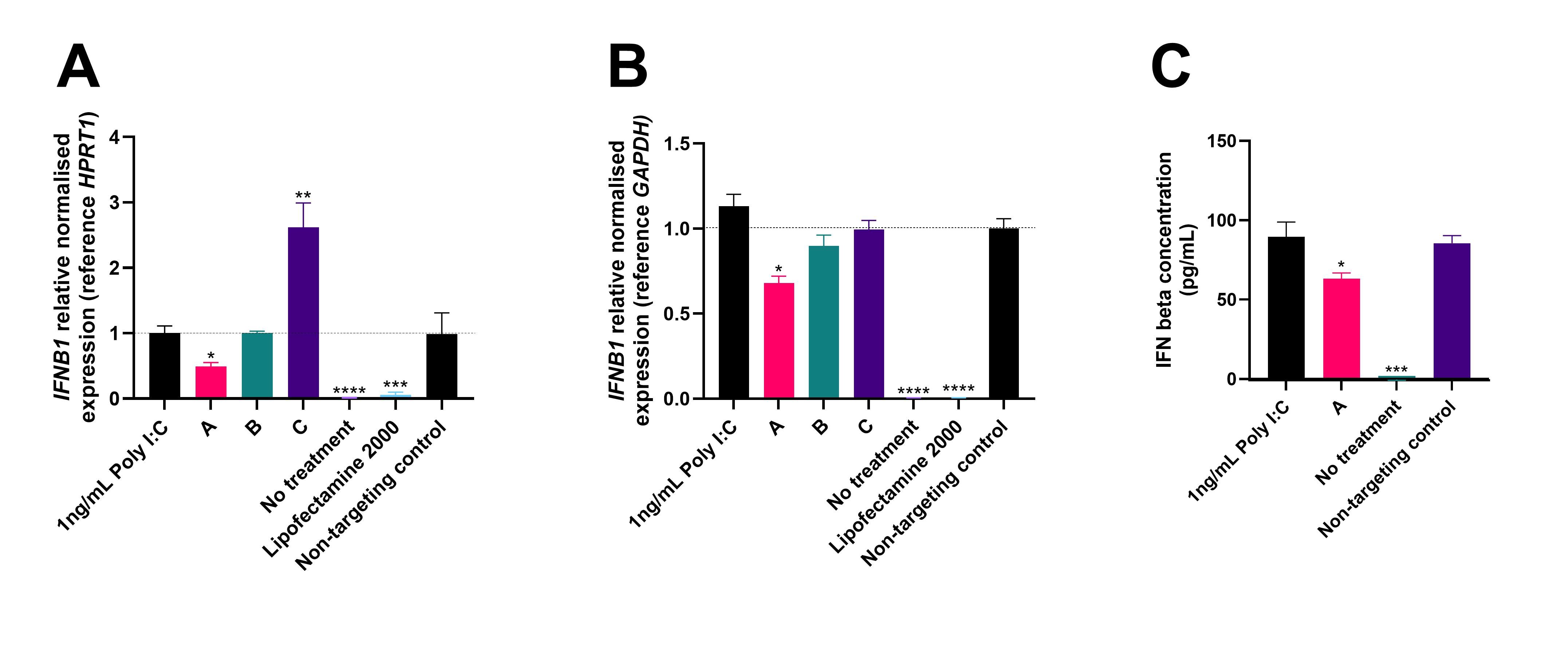
Results: A panel of 30 siRNA was synthesised of which 27 passed quality control and were taken forward into in vitro screening. In myoblasts, Poly I:C treatment induced an 80-fold increase in IFNB1 expression compared to untreated cells at 72 hours. Treatment with the panel of siRNA for 48 hours produced a range of responses including no change, significant upregulation and significant knockdown of IFNB1 mRNA expression (Figure 1A). Some of these siRNAs were taken forward for testing in differentiated myotubes. The Poly I:C treatment induced a greater response in myotubes with a 246-fold increase in IFNB1 expression (Figure 1B). Some siRNAs showed significant knockdown in both myoblasts and myotubes. An ELISA using cell culture media collected from the myotube screening showed that siRNA demonstrating significant knockdown of IFNB1 transcripts also had significantly reduced levels of secreted IFN beta protein (Figure 1C).
Conclusion: Initial in vitro screening demonstrates that siRNA can knockdown IFNB1 and thereby reduce the levels of IFN beta protein secreted by muscle cells. Knockdown of IFNB1 using siRNA is a promising approach as a novel therapeutic in DM/JDM and other diseases with elevated IFN beta.
Immortalised myoblasts and differentiated myotubes were transfected with 1ng/mL Poly I:C for 24 hours to induce IFN beta expression and then transfected with siRNA for 48 hours. Normalised relative expression of IFNB1 transcript in myoblasts (A) and myotubes (B) with reference gene HPRT1 or GAPDH, compared to non-targeting control siRNA. Values calculated using Bio-Rad CFX Maestro software. Levels of secreted IFN beta in the cell media of myotubes measured by ELISA (C). Concentration calculated using an IFN beta standard curve, ANOVA performed, and figures generated using GraphPad Prism 10 software.
* Indicates p-value <0.05, **<0.01, ***<0.001, ****<0.0001
To cite this abstract in AMA style:
Parkes J, Correa-Sánchez A, Cunningham M, Oliver P. In Vitro Screening of siRNAs Designed to Knockdown Interferon Beta as a Novel Therapeutic Approach for Treatment of Adult and Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/in-vitro-screening-of-sirnas-designed-to-knockdown-interferon-beta-as-a-novel-therapeutic-approach-for-treatment-of-adult-and-juvenile-dermatomyositis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-vitro-screening-of-sirnas-designed-to-knockdown-interferon-beta-as-a-novel-therapeutic-approach-for-treatment-of-adult-and-juvenile-dermatomyositis/