Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
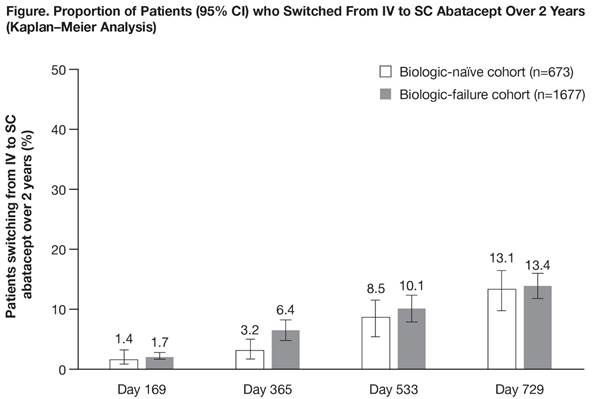
Results: In total, 2350/2364 pts (99.4%) were evaluable for this analysis (673 [28.6%] biologic naïve, 1677 [71.4%] biologic failure); 728 (43.4%) biologic-failure pts had received 1, and 949 (56.6%) had received ≥2 previous biologics. Baseline characteristics in biologic-naïve and biologic-failure pts, respectively, were: mean (SD) age 59.9 (12.7) and 56.9 (12.5) years; mean (SD) RA duration 7.2 (8.22) and 12.1 (9.13) years; 496 (73.7%) and 1379 (82.2%) were women; 621 (92.3%) and 1552 (92.5%) had received prior MTX; and 533 (79.2%) and 1386 (82.6%) had received corticosteroids. Over 2 years, 195 pts switched from IV to SC abatacept (57 biologic naïve, 138 biologic failure; Figure). The primary reason for switch was pt wish (in 28/51 [54.9%] biologic-naïve and 75/121 [62.0%] biologic-failure pts with reason for switch available). Only 8 pts (4.1%) re-switched to IV abatacept (2 biologic naïve, 6 biologic failure). Reasons for re-switch were: pt wish (n=4), lack of efficacy (n=4), safety issue (n=1) and other (n=2). Clinical efficacy outcomes, stratified by prior treatment history, were generally similar at last follow-up before switching from IV to SC abatacept and at last follow-up on SC abatacept treatment (Table).
Conclusion: Over 2 years, in real-world clinical practice, of the pts who switched from IV to SC abatacept, less than 5% re-switched from SC abatacept to the IV formulation. No adverse clinical impact was observed following switching from IV to SC abatacept.
1. Keystone EC, et al. Ann Rheum Dis 2012;71:857–61.
2. Mueller R, et al. Arthritis Rheumatol 2015;67(Suppl. 10):651–2.
3. Reggia R, et al. J Rheumatol 2015;42:193–5.
4. Monti S, et al. J Rheumatol 2015;42:1993–4.
| |
||||
| |
|
|
||
| |
|
|
|
|
| |
(n=42) |
(n=107) |
(n=34) |
(n=69) |
| |
(n=42) |
(n=107) |
(n=34) |
(n=69) |
| |
(n=40) |
(n=105) |
(n=37) |
(n=69) |
| |
(n=40) |
(n=105) |
(n=37) |
(n=69) |
| |
(n=42) |
(n=107) |
(n=37) |
(n=73) |
| |
(n=39) |
(n=94) |
(n=39) |
(n=72) |
| |
(n=39) |
(n=94) |
(n=39) |
(n=72) |
| |
(n=33) |
(n=86) |
(n=33) |
(n=61) |
| |
(n=33) |
(n=86) |
(n=33) |
(n=61) |
| |
(n=49) |
(n=116) |
(n=44) |
(n=84) |
| |
(n=32) |
(n=87) |
(n=36) |
(n=73) |
| |
(n=40) |
(n=105) |
(n=37) |
(n=69) |
To cite this abstract in AMA style:
Alten R, Lorenz H, Mariette X, Nüßlein H, Galeazzi M, Navarro F, Chartier M, Heitzmann J, Rauch C, Le Bars M. In Real-World Clinical Practice, Patients Switching from IV to SC Abatacept Maintain Clinical Efficacy after Switch [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/in-real-world-clinical-practice-patients-switching-from-iv-to-sc-abatacept-maintain-clinical-efficacy-after-switch/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-real-world-clinical-practice-patients-switching-from-iv-to-sc-abatacept-maintain-clinical-efficacy-after-switch/
