Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic autoimmunity can be driven by monogenic or polygenic risk variants. We aimed to characterize the genetic basis of disease in a family with early-onset autoimmune manifestations including systemic lupus erythematosus (SLE), discoid lupus, and immune thrombocytopenia.
Methods: Whole exome sequencing (WES) was conducted to identify candidate variants. The impact of the identified variants was analyzed by flow cytometry, immunoprecipitation, and luciferase reporter assay in transfected 293T cells. Gene expression in peripheral blood mononuclear cells was profiled by bulk RNA sequencing and plasma cytokines were measured by proximity extension assay.
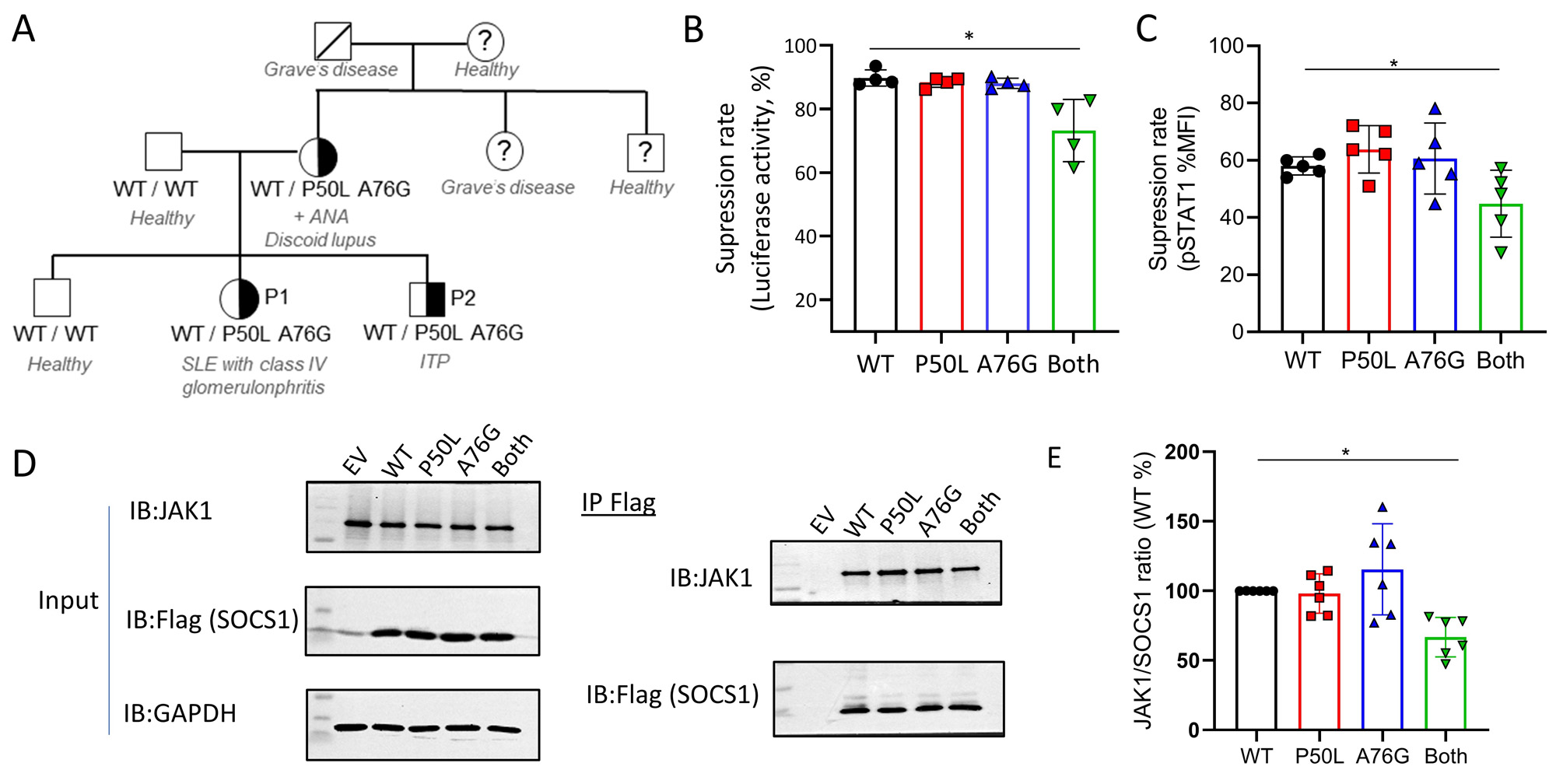
Results: We studied two siblings with early-onset systemic lupus erythematosus and immune thrombocytopenia. Mother of the probands had a history of discoid lupus and positive antinuclear antibody test. WES identified maternally inherited in cis variants (p. Pro50Leu and p.Ala76Gly) in Suppressor of cytokine signaling 1 (SOCS1) flanking the kinase inhibitory domain (Figure 1A). Both variants are predicted to be benign by in silico algorithms and neither variant alone affected the ability of SOCS1 to inhibit JAK-STAT1 signaling by luciferase reporter assay and flow cytometry quantification of STAT1 phosphorylation (Figure 1B,C). Immunoprecipitation studies showed that when both variants were expressed in cis, the mutant SOCS1 protein displayed decreased binding to JAK1 and reduced capacity to inhibit IFN-I signaling by ~25% compared to wildtype protein (Figure 1D,E).
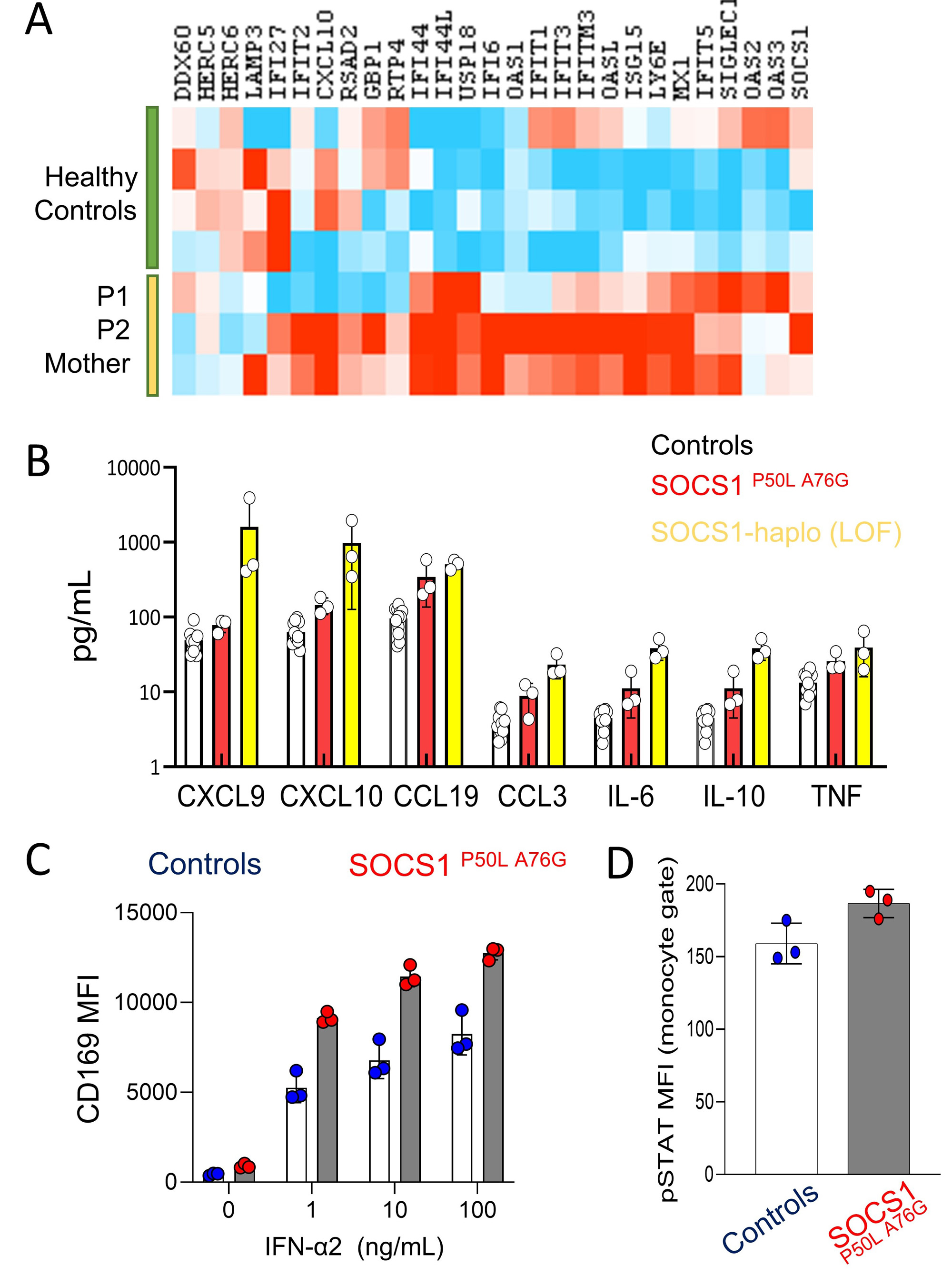
Supporting defective regulation of IFN-I, transcriptomic and cytokine analysis of both siblings and their mother showed increased expression of interferon-inducible genes compared to healthy controls, but less striking compared to patients with SOCS1 haploinsufficiency due to loss-of-function variants (Figure 2A,B). Peripheral blood monocytes from all three subjects further displayed increased CD169 expression and STAT1 phosphorylation upon IFN-I stimulation (Figure 2C,D).
Conclusion: Our work illustrates the critical fine-turning of IFN-I signaling by SOCS1 to prevent autoimmunity and demonstrates that a combination of variants that are individually benign may have deleterious consequences.
To cite this abstract in AMA style:
Du Y, Hsu E, Brodeur K, Liu M, Lo M, Platt C, Lee P. In Cis SOCS1 Variants Illustrate the Precise Regulation of Interferon Signaling Needed to Prevent Autoimmunity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/in-cis-socs1-variants-illustrate-the-precise-regulation-of-interferon-signaling-needed-to-prevent-autoimmunity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-cis-socs1-variants-illustrate-the-precise-regulation-of-interferon-signaling-needed-to-prevent-autoimmunity/