Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Adolescent and young women with rheumatic diseases are often prescribed teratogenic medications to manage their disease. Published reports indicate that the frequency of reproductive health counseling is low in this population, allowing a significant risk of fetal exposure to teratogenic medications. This three-year project aimed to prevent pregnancies in adolescent and young women on teratogenic medications and consisted of two phased initiatives: annual teratogenic medication consents and sexual history documentation at every visit.
Methods: The Institute for Healthcare Improvement model for quality improvement (QI) was used for this project. Each phase included a baseline assessment review and several PDSA cycles. In 2018, annual teratogenic medication consents were created and implemented to facilitate education about teratogenic and fetotoxic risks associated with certain medications. At baseline, there was no consistent documentation of teratogenic medication and/or counseling received by females who were prescribed teratogenic medications. In 2019, we developed sexual history form that was incorporated into the electronic health record to help guide provider discussions with patients and families at every visit and to develop pregnancy prevention plans. Baseline data revealed that none of our female patients who were prescribed teratogenic medications had appropriate sexual history documentation during their clinic visits. To assess the success of reaching our aim of among adolescent and young women using teratogenic medications, we measured days between pregnancies for females on teratogenic medications. In January 2019, days between pregnancies for females on teratogenic medications was determined to be 42 days.
Results: The percentage of females that received annual teratogenic consents increased from 0% to 85% after 18 months. After 8 months, the proportion of female patients that had appropriate sexual history documentation at every visit increased from 0% to 35%. For females on teratogenic medications, the days between reported pregnancies increased from 42 days between pregnancies in January 2019 to >340 days in June 2020.
Conclusion: The implementation of a phased QI project to prevent pregnancies among adolescent and young women using teratogenic medications has been successful within our institution. Coming initiatives, such as routine pregnancy screening and ongoing pregnancy-prevention plans, will help to prevent exposure of teratogenic medications to pregnant females and enhance safe use of these medications. We hope expand aspects of this project to other divisions within our institution and potentially across institutions, as well.
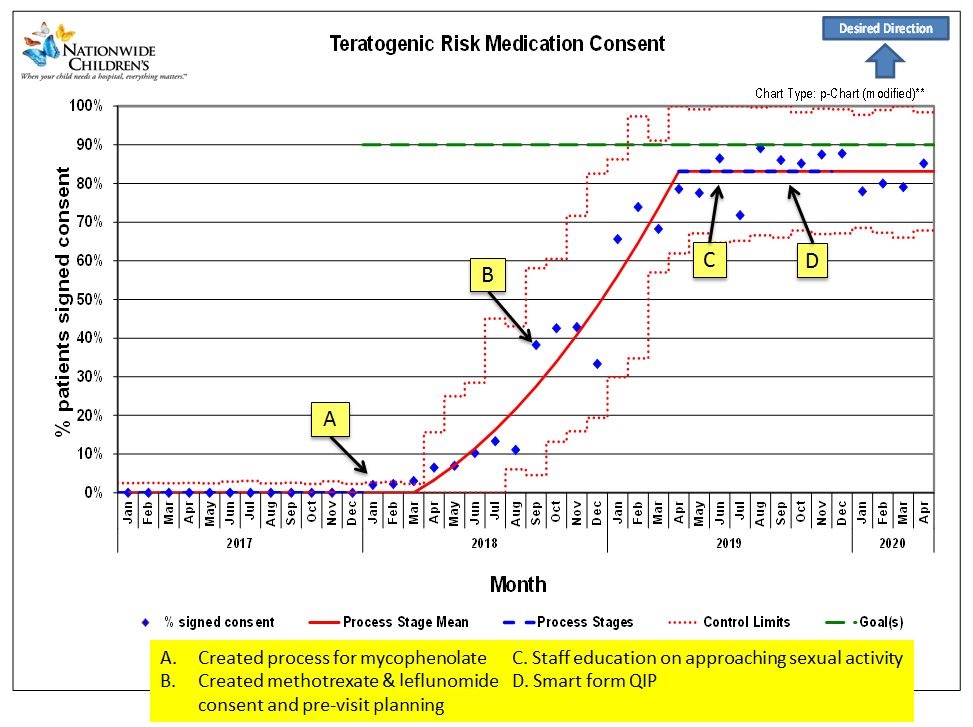 P-chart for teratogenic risk medication consent for all females patients 12 years and older on teratogenic medications with annotated QI PDSA cycle interventions.
P-chart for teratogenic risk medication consent for all females patients 12 years and older on teratogenic medications with annotated QI PDSA cycle interventions.
 Run chart for sexual history documentation within electronic health record smartform at each visit for all patients on teratogenic medications age 12 years and older with annotated QI PDSA interventions.
Run chart for sexual history documentation within electronic health record smartform at each visit for all patients on teratogenic medications age 12 years and older with annotated QI PDSA interventions.
 G-chart indicating days between pregnancies for patients on teratogenic medications followed in rheumatology clinic.
G-chart indicating days between pregnancies for patients on teratogenic medications followed in rheumatology clinic.
To cite this abstract in AMA style:
Mruk V, Wise K, Ardoin S, Oberle E, Lemle S, Sivaraman V, Driest K, Berlan E, Yildirim-Toruner C, Maher J, Jones S, Barbar-Smiley F. Improving Teratogenic Medication Consent and Sexual Activity Screening in Adolescent and Young Females: A Pediatric Rheumatology Reproductive Health Initiative [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/improving-teratogenic-medication-consent-and-sexual-activity-screening-in-adolescent-and-young-females-a-pediatric-rheumatology-reproductive-health-initiative/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-teratogenic-medication-consent-and-sexual-activity-screening-in-adolescent-and-young-females-a-pediatric-rheumatology-reproductive-health-initiative/
