Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The Centers for Disease Control and Prevention and expert rheumatologists recommend screening for HBV and HCV prior to DMARD initiation and the ACR recommends screening for TB prior to biologic initiation. In our study, we aimed to improve patient safety by closing care gaps in the screening laboratories of our patients starting new DMARDs via collaboration with our pharmacists.
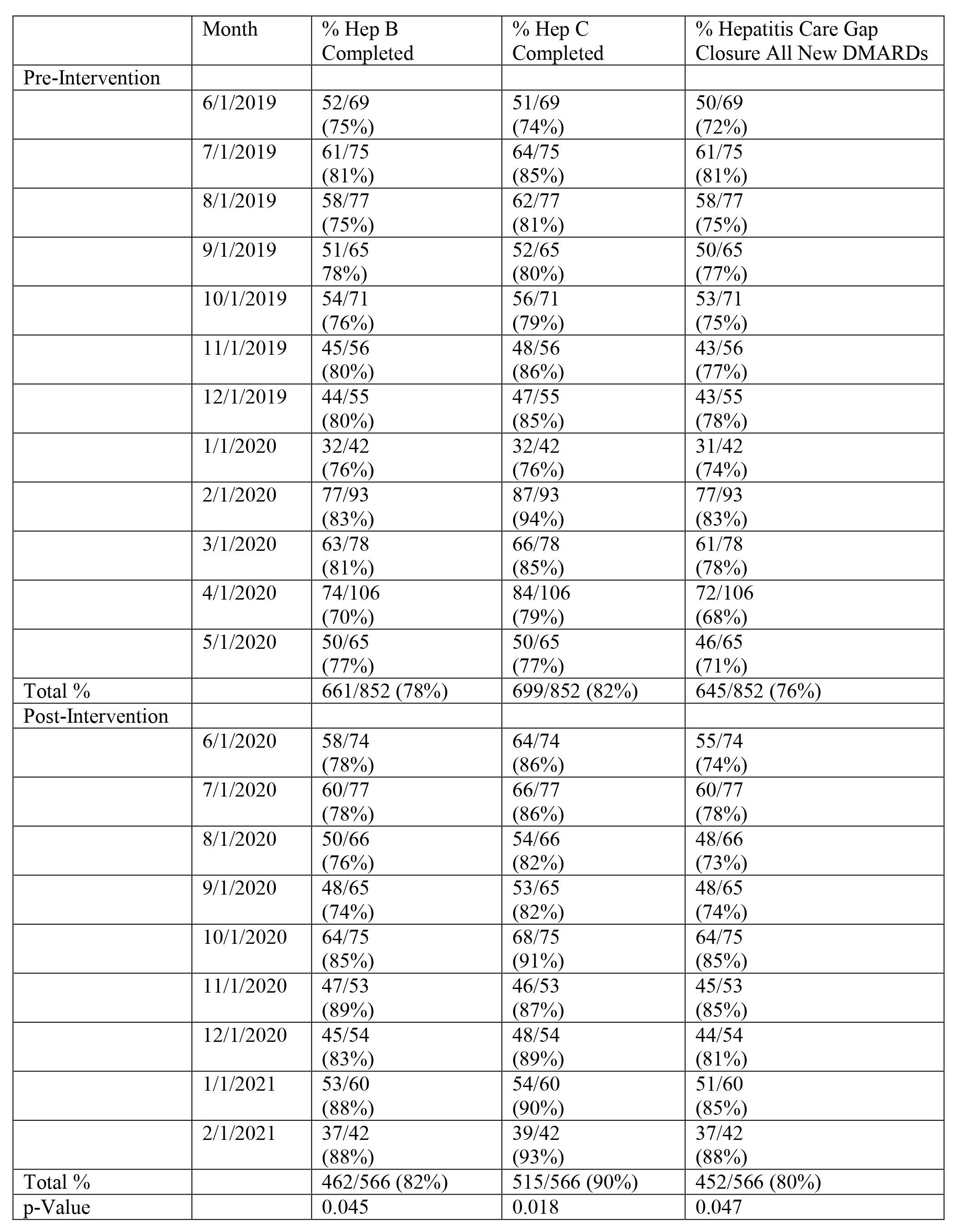
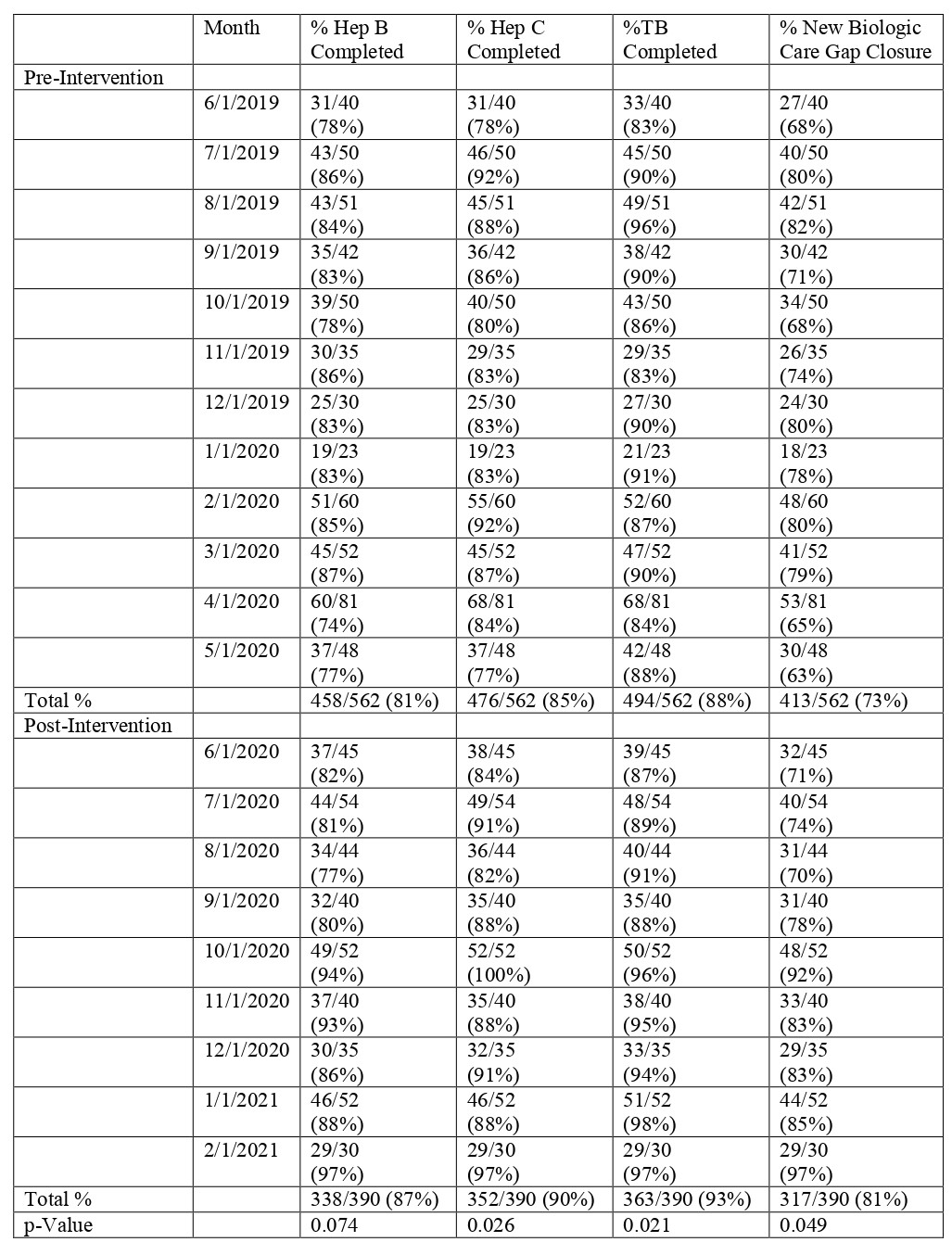
Methods: Hepatitis care gap closure for all patients starting a new DMARD was defined as meeting laboratory screening criteria for both HBV (HBsAg and HBcAb, at least IgM) and HCV (HCV Antibody or quantitative RNA). Additionally, TB screening (Purified Protein Derivative or QuantiFERON) was required to close the care gap in patients starting a new biologic. The study intervention consisted of Rheumatology pharmacist co-management protocols for all DMARDs and a pre-authorization smart-set with screening laboratory checks for new biologics. Data was extracted from our electronic health record (EHR) for patients with completed Rheumatology visits starting a new DMARD 1/1/2019 – 5/31/2020 at baseline and 6/1/2020 – 2/28/2021 post-intervention with pharmacist/physician collaborative screening. Screening was counted as present if there was documentation of completed tests in the chart any time prior to the DMARD initiation and up to one month after. For all new DMARD starts, the percent of HBV and HCV screened patients was calculated respectively. The hepatitis care gap closure for all new DMARDs was defined as meeting both HBV and HCV screening in this population. For all new biologic starts, the percent of HBV, HCV, and TB screened patients was calculated respectively. The care gap closure for new biologics was defined as meeting all three components. Two-sample t-tests were used to measure statistical significance of our screening laboratories.
Results: New DMARD hepatitis screening and new biologic hepatitis/TB screening were examined in 852 and 562 patients at baseline and 566 and 390 patients respectively post-intervention. HBV and HCV screening for all DMARDs increased from 78% and 82% at baseline to 82% (p=0.045) and 90% (p=0.018) respectively post-intervention (Table 1). The care gap closure for total hepatitis screening in all patients starting new DMARDs increased from 76% to 80% (p=0.047) post-intervention (Table 1). For patients starting new biologics, TB screening increased from 88% to 93% (p=0.021) post-intervention while the average care gap closure for hepatitis and TB screening increased from 73% to 81% (p=0.049) post-intervention (Table 2). Figure 1 shows the improvement in care gap closure over time for all new DMARD and all new biologic starts respectively, from baseline through intervention.
Conclusion: Avoiding harm to patients from the care that is intended to help them is essential. Using Rheumatology pharmacists to follow protocols driven by EHR tools, we have significantly improved our patient screening for hepatitis B, hepatitis C, and TB in DMARD-treated rheumatology patients. Today we are one step closer to fulfilling one of the aims of the framework set forth by the Institute of Medicine: Providing safer care for our rheumatic population.
To cite this abstract in AMA style:
Nicholas P, Cote J, Grassi D, Thomas S, Chronowski J, Pugliese D, Newman E. Improving Safety in Rheumatology Patients by Closing Pre-screening Laboratory Care Gaps [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improving-safety-in-rheumatology-patients-by-closing-pre-screening-laboratory-care-gaps/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-safety-in-rheumatology-patients-by-closing-pre-screening-laboratory-care-gaps/