Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Biologics and small molecules (bDMARDs) are important immunomodulatory medications for management of patients with rheumatic diseases. Use of a bDMARD in patients with infection with pre-existing tuberculosis (TB), hepatitis B (HBV), or hepatitis C (HCV) can have serious consequences. Many national and international societies recommend timely screening for one or all of these exposures prior to initiation of certain bDMARDs, however, adherence to these recommendations varies widely. This quality improvement initiative focused on improving compliance with current standards using a best practice advisory (BPA) in the electronic health records (EHR).
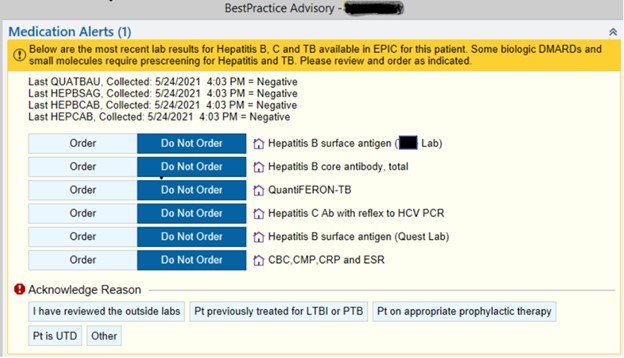
Methods: Patients aged 18 years or older with at least two visits with a provider in the section of Rheumatology in the designated time frame were included. Upon a new prescription of a bDMARD, providers were alerted via a BPA pop-up in the EHR about the last available results if any, for TB, HBV, and HCV, and allowed for quick ordering as required. This BPA was implemented starting in December 2020. To assess the impact of the BPA, baseline rates of screening for TB (Quantiferon), HBV, and HCV for patients prescribed a new bDMARD from October 1, 2017 to November 30, 2020 (pre-BPA) were compared with those of patients with prescriptions from December 1, 2020 to April 9, 2021 (post-BPA). Data were collected for various bDMARDs targeting tumor necrosis factor (TNF), interleukin-17 (IL-17), interleukin-6 (IL-6), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), CD20, and Janus kinase (Jak).
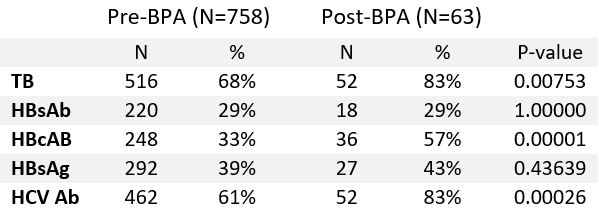
Results: A total of 821 patients met the above inclusion criteria with 768 patients pre-BPA and 63 patients post-BPA implementation. The two patient populations were similar in gender, age, race/ethnicity, rheumatic disease diagnosis, and a new bDMARD prescription. Median age of study population was 65 years, 70% were female, majority (77%) were white; most common rheumatic diseases were inflammatory arthritis (88%) and connective tissue disease (7%). TNFi (52%), IL-17 inhibitors (16%) and JAK inhibitors (15%) were most common bDMARDs prescribed. BPA implementation was associated with statistically significant improvement in screening for TB (pre-BPA 68% vs post-BPA 83% p=0.001), HCV (61% vs 83% p=0.0003) and hepatitis B core antibody (HBcAb) (33% vs 57% p=0.00001), numerical improvement in hepatitis B surface antigen (HBsAG) screening (39% vs 43% p=0.44) and no improvement in hepatitis B surface antibody (HBsAb) testing (29% each p=1.0) (Table 1).
Conclusion: Implementation of an electronic BPA in the EHR has the capacity to improve screening rates in patients who are started on a bDMARD. The BPA facilitates rapid review of screening tests and enhances provider experience and efficiency. Limitations include difficulty in capturing laboratory results performed outside the EHR.
To cite this abstract in AMA style:
Baker H, Fine R, Hsiao B, Chowdhary V, Suter L, Danve A. Improving Pre-biologic Infection Screening Using a Best Practice Alert in Electronic Health Records [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improving-pre-biologic-infection-screening-using-a-best-practice-alert-in-electronic-health-records/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-pre-biologic-infection-screening-using-a-best-practice-alert-in-electronic-health-records/