Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Hypogammaglobulinemia following rituximab therapy is a potentially severe complication that can lead to infection-related morbidity and mortality. If recognized, clinicians may prevent infections by prescribing intravenous or subcutaneous immune globulin. Our goal was to improve hypogammaglobulinemia monitoring and documentation in pediatric rheumatology patients treated with rituximab therapy.
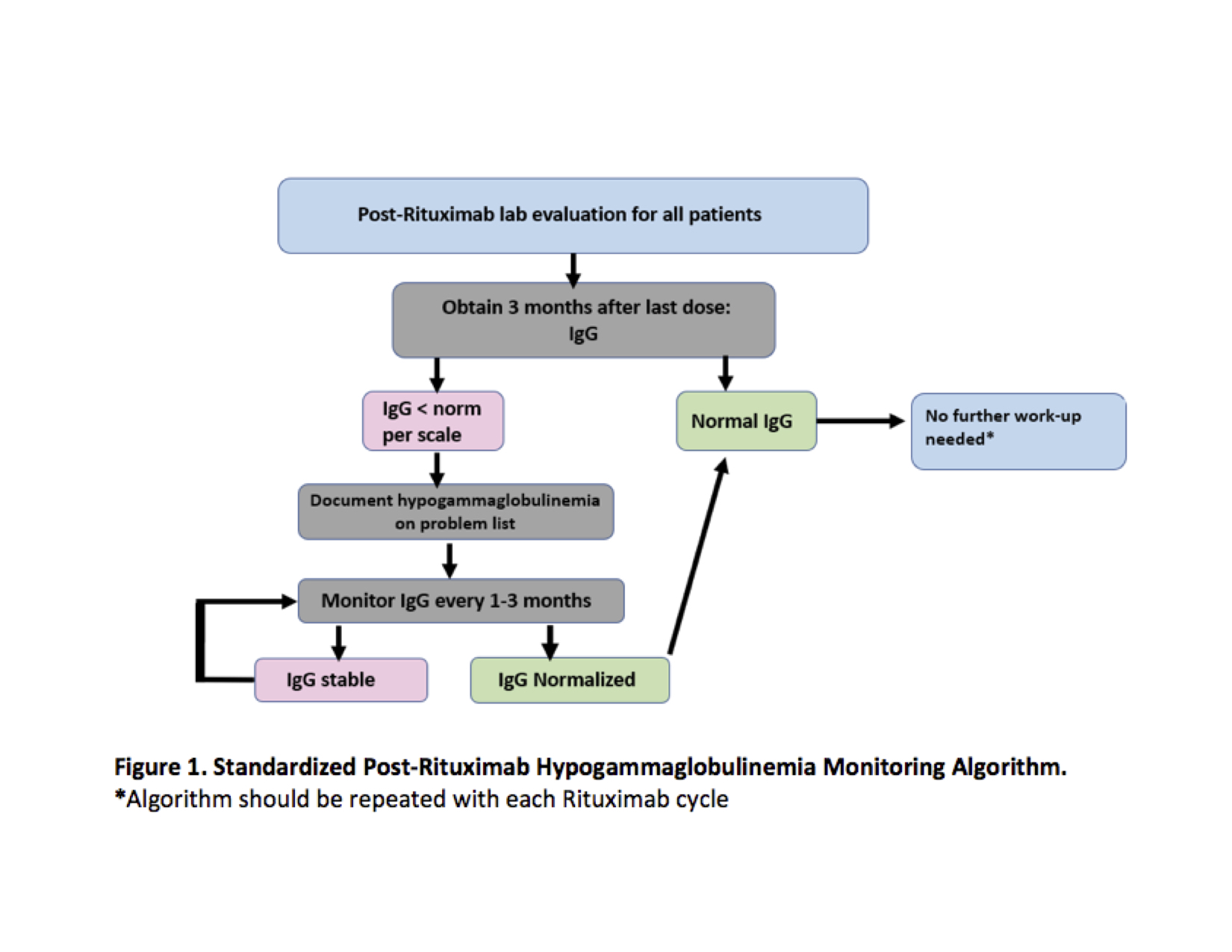
Methods: In September 2020, we identified patients prescribed rituximab within the previous 24 months and had a Rheumatology visit within the previous 6 months. We identified inconsistent IgG monitoring amongst providers and non-standardized documentation of hypogammaglobulinemia when identified. We then developed an IgG monitoring process based on adult guidelines and pediatric literature (Figure 1).
Our process measure, hypogammaglobulinemia risk assessment, was defined as 1) IgG testing based on algorithm recommendations, and 2) hypogammaglobulinemia documentation in the electronic health record problem list if IgG was low per scale. We aimed to increase the number of clinic visits fulfilling hypogammaglobulinemia risk monitoring between missed opportunities (goal > 6) by June 30, 2021. The outcome measure was the proportion of eligible patients with up-to-date hypogammaglobulinemia risk assessment (goal > 80%).
We conducted the following “Plan-Do-Study-Act” (PDSA) cycles, beginning in September 2020: 1) pilot testing of algorithm, 2) division-wide dissemination of algorithm and provider-specific patient lists specifying those overdue for monitoring and/or documentation, and 3) education update reviewing divisional progress towards goals, algorithms, and provider-specific patient lists.
Data were analyzed for special cause variation using standard statistical process control and run chart methodology. Patients who received IgG replacement within the last 6 months, had transferred to an alternative practice, or were lost to follow-up (no visit within 12 months) were ineligible.
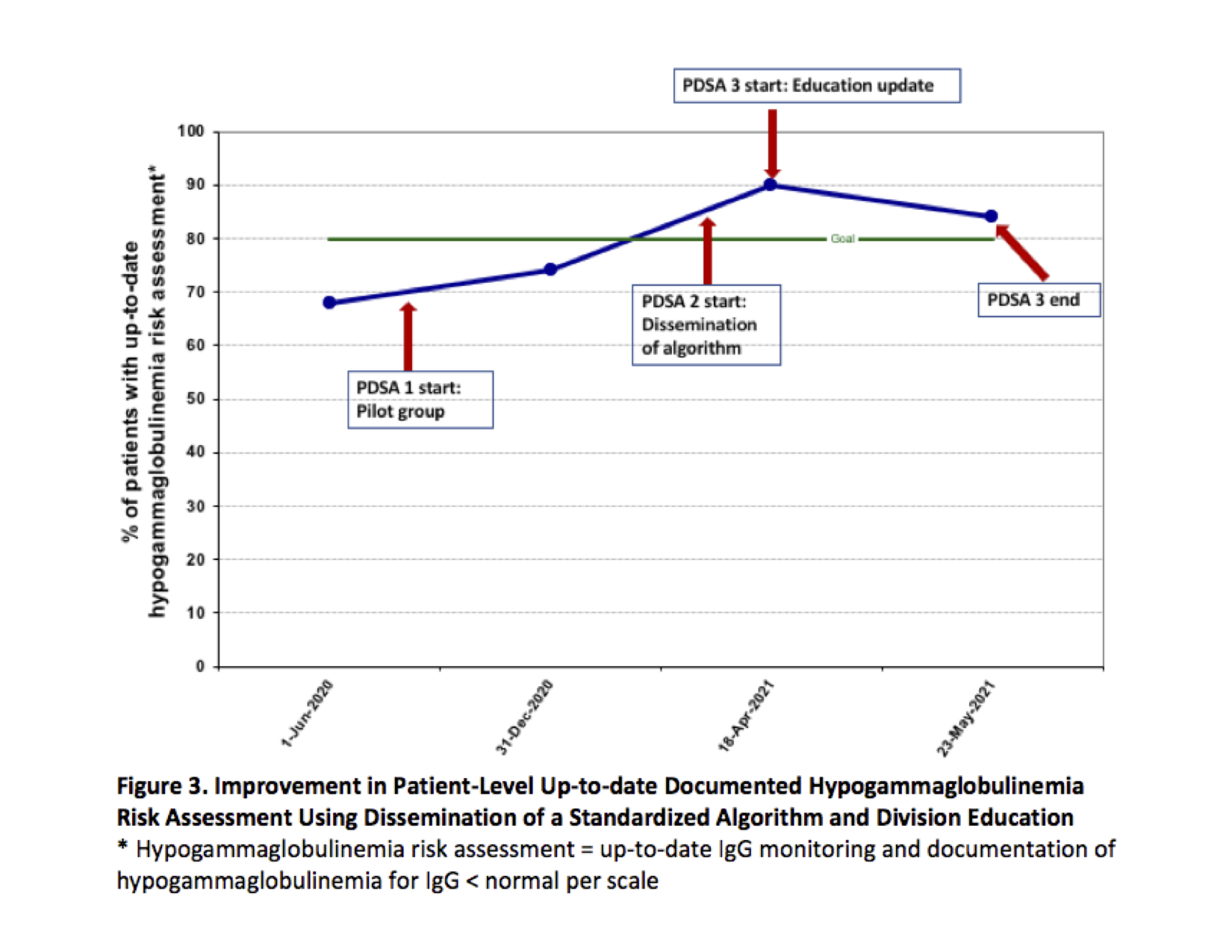
Results: Beginning in May 2020, the baseline median number of visits between missed hypogammaglobulinemia risk assessment opportunities was 1.18. Special cause variation criteria were met after the first PDSA cycle, with an increase in successful monitoring opportunities to 5.17 (Figure 2). Currently, we have successfully monitored 14 patients consecutively. Patients with an up-to-date clinical risk assessment increased from 68% (26/38) to 84% (21/25) (Figure 3). Documentation of identified hypogammaglobulinemia increased from 38% to 100%.
Conclusion: Provision of a standardized algorithm paired with intermittent provider-specific reminders improves identification and documentation of hypogammaglobulinemia in rheumatology patients who have received rituximab. We are nearing our risk assessment goal and will evaluate whether our improvements are sustained. Future directions include standardizing referrals to immunology for replacement immunoglobulin therapy and developing clinical decision support tools.
To cite this abstract in AMA style:
Rutstein B, Argraves M, Bilgic Dagci A, Bayefsky S, Rood J, Chase J, Mehta J, Lerman M, Stingl C, Burnham J. Improving Post-Rituximab Hypogammaglobulinemia Risk Assessments: A Fellows’ Quality Improvement Initiative [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improving-post-rituximab-hypogammaglobulinemia-risk-assessments-a-fellows-quality-improvement-initiative/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-post-rituximab-hypogammaglobulinemia-risk-assessments-a-fellows-quality-improvement-initiative/