Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Session Type: Poster Breakout Session
Session Time: 4:30PM-5:00PM
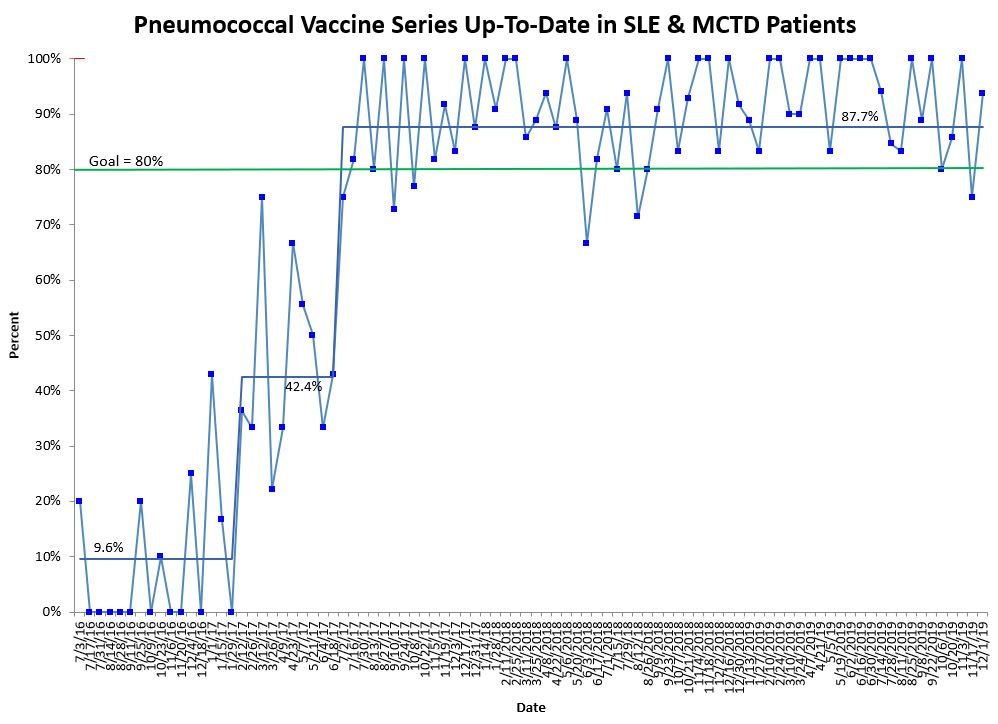
Background/Purpose: The Centers for Disease Control and Prevention recommends pneumococcal vaccination of high-risk patients, including patients on iatrogenic immunosuppression. Many patients seen in the rheumatology clinic are at increased risk of invasive pneumococcal infection secondary to primary or acquired immunodeficiencies. A quality improvement project to increase pneumococcal vaccination rates in patients with systemic lupus erythematosus (SLE) and mixed connective tissue disease (MCTD) was started in September 2016. After increasing the combined pneumococcal vaccination rate to 87.7% for those with SLE and MCTD, our new aim in July 2018 was to have 80% of patients on immunosuppression with SLE, MCTD, juvenile dermatomyositis (JDM) and systemic vasculitis up-to-date on pneumococcal vaccinations by July 2019.
Methods: Our division established a project team and utilized quality improvement methodology including a driver diagram, process map, fishbone diagram, and plan-do-study-act cycles. Two process measures were created: 1) being up-to-date on the 13-valent pneumococcal conjugated vaccine (PCV13) and 2) being up-to-date on the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Our outcome measure was being completely up-to-date on pneumococcal vaccinations. Eligible patients from September 2016 to June 2018 were all SLE and MCTD patients. From July 2018 onward, JDM and vasculitis patients on immunosuppression were eligible along with all SLE and MCTD patients. Interventions included: generating an immunization algorithm, creating a report of eligible patients, providing physician and nurse education, sending email reminders, and performing pre-visit planning. Control charts were made to evaluate for change over time.
Results: Data collection of pneumococcal immunization rates began in July 2016 and is ongoing. There were shifts in the center line for all three quality measures in both phases of our project indicating a significant increase in vaccination rates. Average PCV13 rate for SLE and MCTD patients increased from 32.1% to 91.5%, and PPSV23 rate went from 17.1% to 89.9%. Combined pneumococcal vaccination rate for SLE and MCTD patients increased from 9.6% to 87.7%, and this mean has been sustained for over two years (Figure 1). Pneumococcal vaccination rates also significantly increased for phase 2 with SLE, MCTD, JDM, and vasculitis patients: 70.5% to 92.2% for PCV13, 63.8% to 86% for PPSV23, and 62.6% to 86% for the combined pneumococcal vaccination rate (PCV13 and PPSV23) (Figure 2). There have been no significant known adverse events related to the vaccinations.
Conclusion: Using quality improvement methodology, pneumococcal vaccination rates significantly increased and have been sustained in our immunosuppressed pediatric rheumatology patients. We continue to prioritize this important initiative through a multi-specialty project and through pre-visit planning by identifying additional rheumatology patients on immunosuppression that are eligible for pneumococcal vaccination.
To cite this abstract in AMA style:
Harris J, Holland M, Fox E, Ivy A, Ibarra M, Hoffart C, Jones J, Favier L, Cooper A. Improving Pneumococcal Vaccination Rates in Immunosuppressed Rheumatology Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/improving-pneumococcal-vaccination-rates-in-immunosuppressed-rheumatology-patients/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-pneumococcal-vaccination-rates-in-immunosuppressed-rheumatology-patients/