Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Musculoskeletal (MSK) manifestations of SLE are the most frequently assessed domain in clinical trials. Optimal MSK assessment is contested; candidates include the binary or ordinal SLEDAI and BILAG, tender joint count (TJC), swollen joint count (SJC) and “active” joint counts, (either tender or swollen, or both).Ultrasound studies in SLE have identified imaging-only synovitis, such that the BILAG A/B criterion has a sensitivity of just 56% for US synovitis.Our aim was to assess sensitivity and specificity of a set of candidate joint counts against US synovitis, as well as patient and US defined response to therapy.
Methods: We performed a post-hoc analysis of a multi-centre study [USEFUL; PMID: 33792659]. Patients with inflammatory arthralgia were assessed clinically and using US at 0, 2 and 6 weeks following single-dose intra-muscular glucocorticoid therapy.Candidate joint sets were defined as: 68 TJC and SJC, 28 TJC and SJC, or 24 TJC and SJC (MCPs, PIPs, elbows, wrists). Joint counts were compared with weighted scatter plots and intraclass correlation coefficients (ICCs).Participant-reported change was measured using a Likert scale (-7 to 7). For US response, changes in number of ‘active’ joints (grey scale ≥2 and/or power Doppler ≥1) were calculated. Spearman rank correlation measured associations between changes in the candidate joint counts, participant-reported changes and changes in the number of US active joints. Associations are also reported for the composite lupus arthritis and MSK disease activity score (LAMDA) (Table 2).
Results: Of 133 patients, 95% female and 62% European ancestry, 63% had ≥1 SJC at baseline. TJC and SJC had poor sensitivity (50.7% and 41.6% respectively), and TJC had lower specificity than SJC (60.7% and 91.4% respectively)(Table 1). Active “either” and active “both” almost exactly matched TJC and SJC respectively (Figure 1a-h). In joint-by-joint analysis the most commonly swollen joints were MCPs 2-5.Tenderness was most common in MCP 2 and 3, wrist, shoulder and knee. Reduced joint counts were more strongly correlated with US-defined response than full joint counts (Figure 1i) but not with participant-defined response. Changes in TJCs correlated with participant-defined response and changes in SJCs with US-defined response but not vice-versa. LAMDA was modestly correlated with both response definitions.
Conclusion: (1) TJC and SJC have limited sensitivity for imaging-detected synovitis, but SJC has high specificity. (2) Active ‘either’ and ‘both’ joint counts almost exactly match TJC and SJC respectively and are therefore redundant. (3) Diagnostic accuracy of clinical assessment varies by joint area; shoulders and knees likely contribute little to assessment of joint inflammation and often have non-inflammatory pathology. (4) SJC is the most responsive measure of objective synovitis; SJC24 is the most responsive set for US response, but is not correlated with participant-defined response. Given the relative insensitivity of SJC, and importance of patient defined response, the composite measure, LAMDA may be optimal in RCTs. Future work will evaluate these variables in randomised trials and define inclusion and response thresholds.
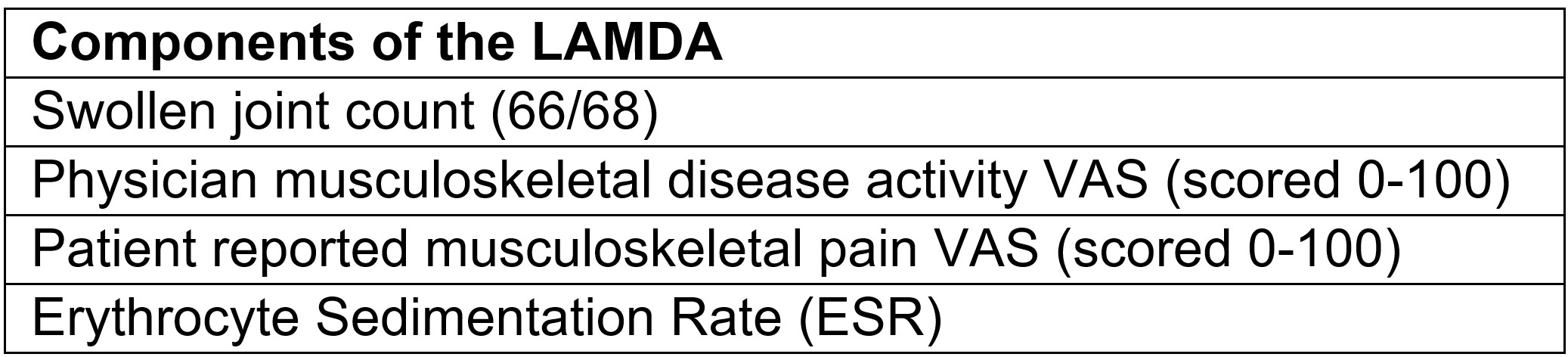 Table 1. Sensitivity and specificity of clinical assessment in the detection of ultrasound-detected synovitis.
Table 1. Sensitivity and specificity of clinical assessment in the detection of ultrasound-detected synovitis.
.jpg) Figure 1 a-h: Weighted scatter plots of joint counts versus active definitions at baseline. Intraclass correlation coefficients (ICCs) displayed; i: Associations between changes in joint assessments and participant- or US-defined response at 6 weeks.
Figure 1 a-h: Weighted scatter plots of joint counts versus active definitions at baseline. Intraclass correlation coefficients (ICCs) displayed; i: Associations between changes in joint assessments and participant- or US-defined response at 6 weeks.
.jpg) Components of the composite lupus arthritis and MSK disease activity (LAMDA) score.
Components of the composite lupus arthritis and MSK disease activity (LAMDA) score.
To cite this abstract in AMA style:
Wood S, Mahmoud K, Md Yusof M, Conaghan P, Hensor E, Vital E. Improving Clinical Outcomes In SLE Arthritis Trials: Post-Hoc Analysis Of A Prospective Intervention Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/improving-clinical-outcomes-in-sle-arthritis-trials-post-hoc-analysis-of-a-prospective-intervention-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-clinical-outcomes-in-sle-arthritis-trials-post-hoc-analysis-of-a-prospective-intervention-study/
