Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: BIIB059 is a humanized monoclonal antibody against BDCA2, a receptor exclusively expressed on plasmacytoid dendritic cells, which are implicated in the pathogenesis of cutaneous lupus erythematosus (CLE).1,2 Part B of the randomized, Phase 2 LILAC study (NCT02847598) demonstrated a statistically significant dose-response relationship on percent change in Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity (CLASI-A) score from baseline (BL) to Week 16 for BIIB059 vs placebo (PBO) in participants (pts) with active CLE.3 Here, we report additional efficacy outcomes.
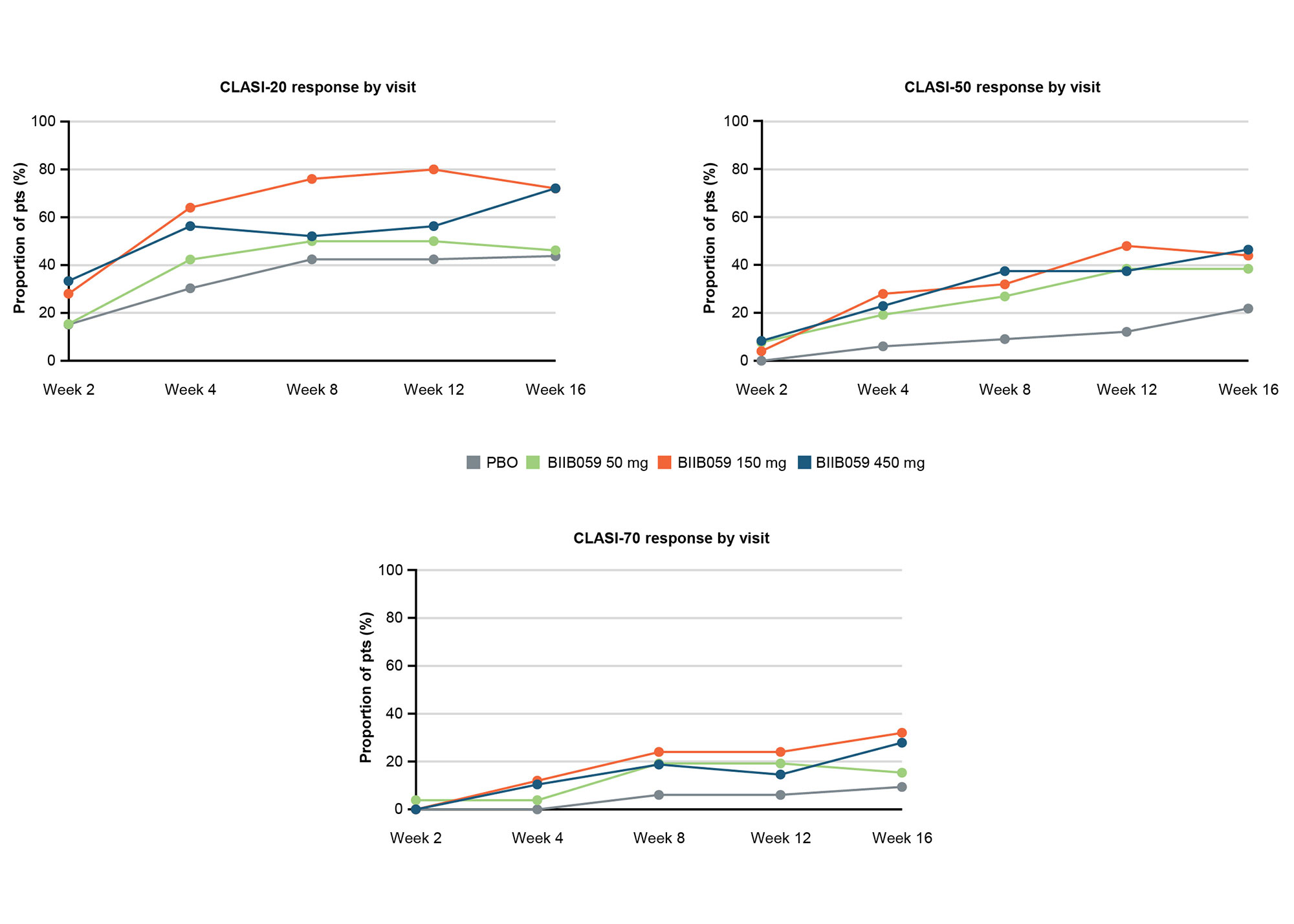
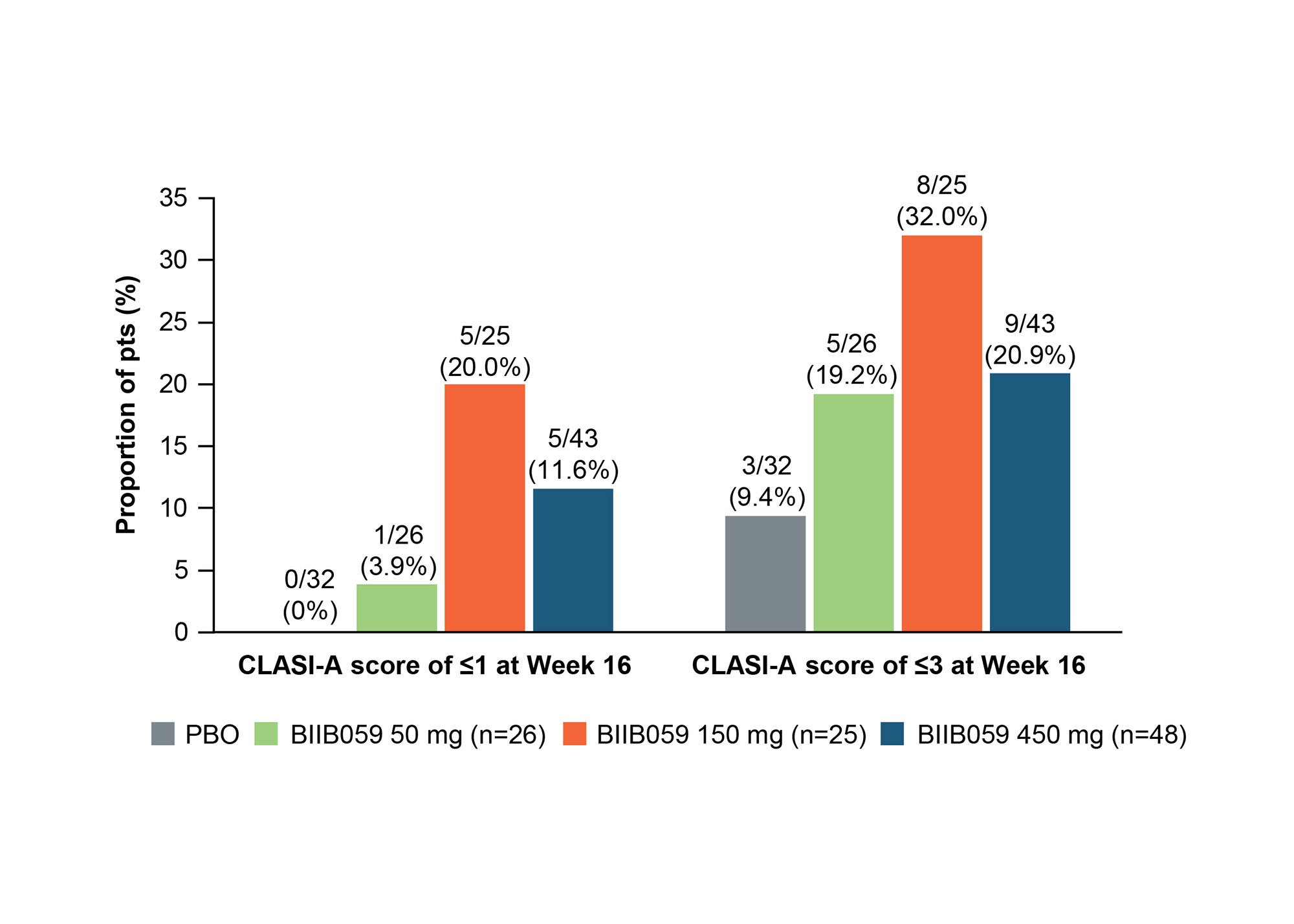
Methods: In LILAC Part B, adults with histologically confirmed active CLE, with or without systemic manifestations, and adjudicated BL CLASI-A scores of ≥8 were randomized to receive BIIB059 50 mg, 150 mg, or 450 mg, or PBO subcutaneously at Weeks 0, 2, 4, 8, and 12. All outcomes were assessed post hoc at Week 16 unless otherwise stated. The proportions of pts achieving 20%, 50%, or 70% reductions in CLASI-A scores from BL (CLASI-20, CLASI-50, and CLASI-70 responses, respectively) were assessed over time, as were those achieving CLASI-A scores of ≤1 (clear skin) or ≤3 (almost clear skin). Improvements from BL to Week 16 in CLASI-A scores were assessed in subgroups with BL CLASI-A scores of < 10 (mild disease) vs ≥10 (moderate/severe disease), and in pts with or without systemic lupus erythematosus (SLE) (prespecified exploratory subgroup analyses). The proportions of pts achieving reductions from BL to CLASI-A scores of ≤1, ≤3, ≤6, and ≤8 were analyzed for the subgroups with BL CLASI-A scores of < 10 and ≥10.
Results: Mean (standard deviation) CLASI-A scores at BL were 15.2 (8.8), 18.4 (8.7), 16.5 (8.8), and 16.5 (8.5) for the BIIB059 50-mg (n=26), 150-mg (n=25), 450-mg (n=48), and PBO (n=33) groups, respectively. Greater proportions of BIIB059-treated pts achieved CLASI-20, CLASI-50, or CLASI-70 responses vs PBO for all doses starting from Week 4 onwards (Figure 1). Although pt numbers were small, greater proportions of BIIB059-treated pts vs PBO achieved CLASI-A scores of ≤1 or ≤3 at Week 16 (Figure 2). All BIIB059-treated groups had greater percent reductions in CLASI-A scores vs PBO at Week 16, regardless of BL score (< 10, n=26 or ≥10, n=106) (Figure 3) or presence of SLE (not illustrated). Higher proportions of pts treated with BIIB059 50 mg, 150 mg, or 450 mg achieved CLASI-A scores of ≤1, ≤3, ≤6, and ≤8 vs PBO at Week 16 regardless of BL score (< 10 or ≥10, not illustrated), with one exception: in the subgroup with BL CLASI-A scores of < 10, two pts in the BIIB059 150 mg group achieved a CLASI-A score of ≤1 at Week 16, vs none in the other groups.
Conclusion: Compared with PBO, greater improvements in skin disease activity were consistently observed in pts treated with BIIB059, independently of disease severity at BL. These results support the continued development of BIIB059 in CLE.
References:
1. Pellerin A, et al. EMBO Mol Med 2015;7:464–476
2. Furie R, et al. J Clin Invest 2019;129:1359–1371
3. Werth V, et al. Ann Rheum Dis 2020;79(Suppl. 1):120–121 (Abs. OP0193)
Funding: This study was funded by Biogen (Cambridge, MA, USA). Writing and editorial support were provided by Selene Medical Communications (Macclesfield, UK), funded by Biogen.
Pts who experienced treatment failure or who discontinued treatment were considered to be non-responders at visits after treatment failure or treatment discontinuation. Pts who completed treatment but had a missing score at any primary timepoint were classified as non-responders for that timepoint. For pts from protocol Version 1 Part B who completed treatment up to Week 12 but could not reconsent to protocol Version 2, Week 16 binary response was derived based on imputed continuous response.
CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; PBO, placebo; pts, participants
Pts who achieved CLASI-A scores of ≤1 are also included in the numbers of pts who achieved CLASI-A scores of ≤3. For pts from protocol Version 1 who completed treatment up to Week 12 but could not reconsent to protocol Version 2, Week 16 data were imputed using the predicted value from the MMRM for the absolute value. The worse observation of BL or last visit before treatment failure carried forward was used to impute values for all visits following treatment failure.
BL, baseline; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity; MMRM, mixed-effect model with repeated measurement; PBO, placebo; pts, participants
Least squares mean (SE) percent change in CLASI-A score from BL to Week 16 was determined using an MMRM with treatment group, study visit, study visit-by-treatment interaction, DLE (presence vs absence), and BL CLASI-A score (≤10 or >10) as fixed covariates. The MMRM used an unstructured covariance structure. For pts considered treatment failures, the worse of BL or last visit before treatment failure carried forward was used to impute values for all visits following treatment failure. Data after pts’ treatment discontinuation are censored. N values represent the modified intent-to-treat population by treatment.
BL, baseline; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity; DLE, discoid lupus erythematosus; LS, least squares; MMRM, mixed-effect model with repeated measurement; PBO, placebo; pts, participants; SE, standard error
To cite this abstract in AMA style:
Werth V, van Vollenhoven R, Furie R, Nyberg F, Kalunian K, Navarra S, Romero-Diaz J, Zhu W, Barbey C, Musselli C, Franchimont N. Improvements in Skin Manifestations with BIIB059 Measured by CLASI in Participants with Cutaneous Lupus Erythematosus (CLE): Exploratory Analyses of the Phase 2, Randomized, Double-Blind, Placebo-Controlled LILAC Study (Part B) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/improvements-in-skin-manifestations-with-biib059-measured-by-clasi-in-participants-with-cutaneous-lupus-erythematosus-cle-exploratory-analyses-of-the-phase-2-randomized-double-blind-placebo-cont/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-skin-manifestations-with-biib059-measured-by-clasi-in-participants-with-cutaneous-lupus-erythematosus-cle-exploratory-analyses-of-the-phase-2-randomized-double-blind-placebo-cont/