Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Guselkumab (GUS) has demonstrated significant efficacy across disease domains in controlled clinical trials of patients (pts) with active PsA.1,2 Recent CorEvitas PsA/SpA Registry analyses confirmed real-world GUS effectiveness, showing that pts with persistent on-label GUS use at 6 months had significant mean improvements from baseline (BL) in peripheral joint and skin symptoms and pt-reported pain.3 Here we report secondary findings from the CorEvitas PsA/SpA Registry further assessing changes in pt-reported outcomes (PROs) among pts with persistent GUS use from BL through the 6-month visit (On-Label GUS Persisters).
Methods: This analysis includes registry pts who initiated on-label GUS after FDA approval for active PsA (7/13/2020; 100 mg SC at Weeks 0 & 4 then Q8W) and were On-Label Persisters. Demographics, PsA disease activity, PROs, and medication history at GUS initiation (BL visit) were summarized descriptively. Among On-Label GUS Persisters not meeting response criteria at BL, response rates at 6 months were determined for established outcomes related to improvements or achievement of low levels of disease activity in pt-reported pain (0-100 mm visual analog scale [VAS]), pt global assessment of arthritis + psoriasis (PtGA; 0-100 mm VAS), and HAQ-DI (0-3). Unadjusted, nominal p-values were calculated using a single-proportion, one-sided test to determine if response at the 6-month visit differed from 0% at BL. Mean (95% CI) change from BL to 6 months was determined for fatigue (0-100 mm VAS); nominal p-value calculated using a paired t-test at α=0.05.
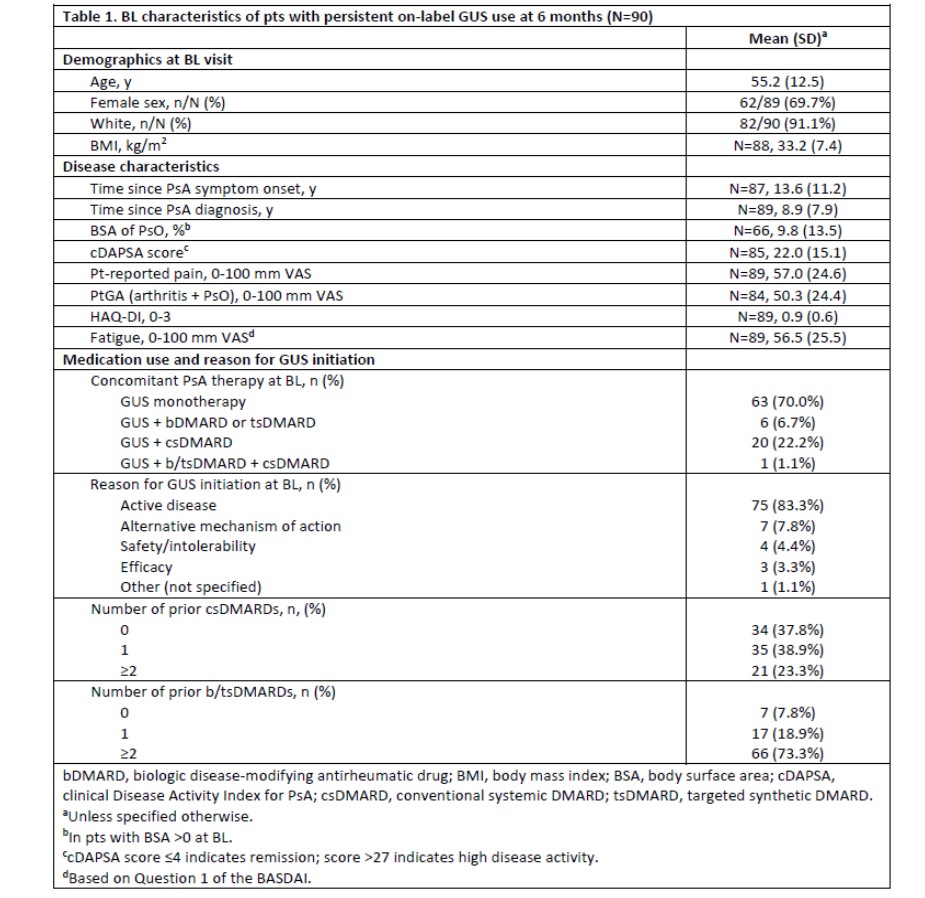
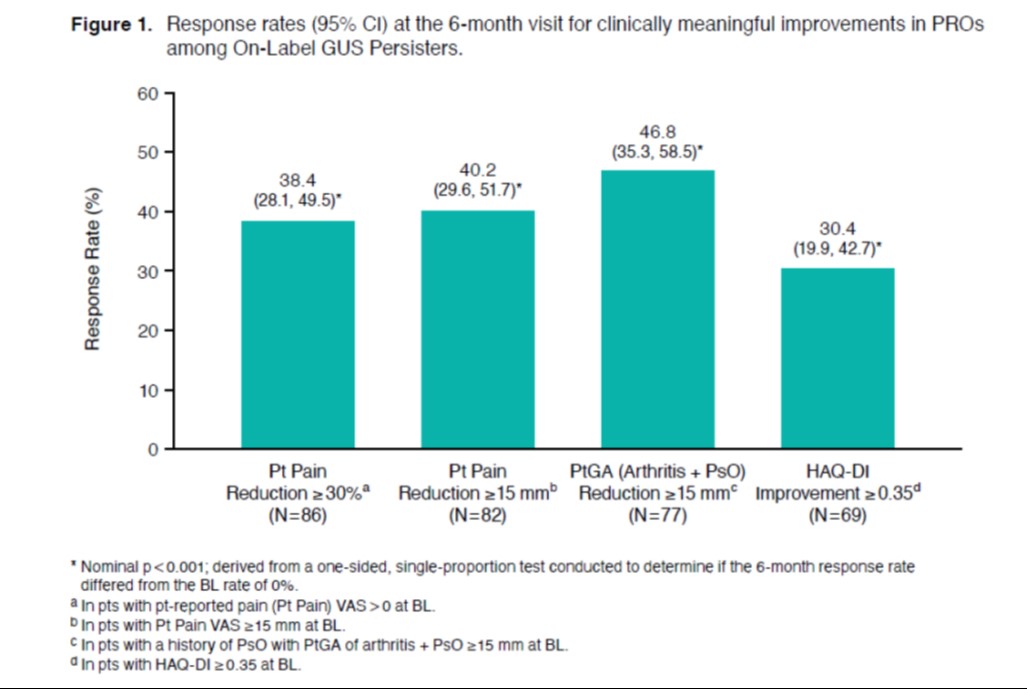
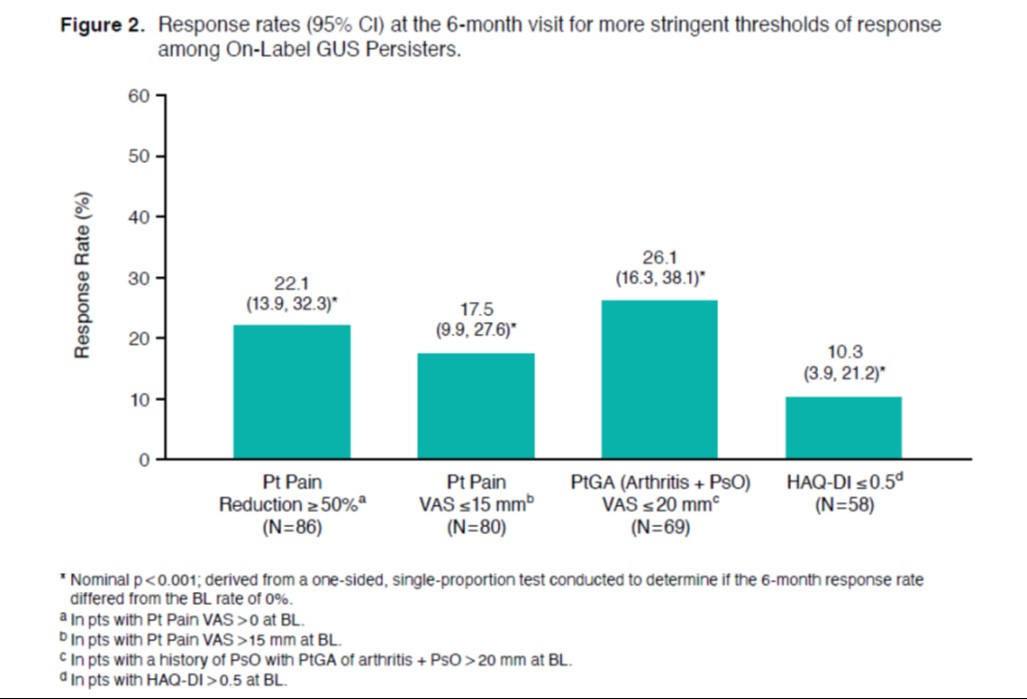
Results: Among 114 on-label GUS initiators with a 6-month follow-up visit, 90 (79%) had persistent on-label GUS use. On average, pts had longstanding, treatment-resistant, active PsA (Table 1). BL pt-reported pain, HAQ-DI, and fatigue scores were 57.0, 0.9, and 56.5, respectively. Substantial proportions of On-Label GUS Persisters experienced clinically meaningful improvements in pain (38% with ≥30% reduction and 40% with ≥15-mm reduction), overall joint and skin disease (47% with ≥15-mm reduction in PtGA), and physical function (30% with HAQ-DI improvement ≥0.35; all nominal p< 0.001; Figure 1). Further, up to one quarter of pts achieved the more stringent thresholds of response, generally representing a major response or minimal disease activity, including 22% with ≥50% reduction in pain, 18% with pain score ≤15 mm, 26% with PtGA score ≤20 mm, and 10% with HAQ-DI ≤0.5 (all nominal p< 0.001; Figure 2). Mean change (95% CI) in fatigue from BL at 6 months was -8.8 (-14.9, -2.7; nominal p=0.005).
Conclusion: In this real-world population of pts with treatment-resistant active PsA and persistent on-label GUS use, consistent with prior results for physician-reported endpoints of joint and skin disease, pts reported meaningful improvements in pain, physical function, and fatigue. These represent difficult-to-treat domains that are important contributors to pts’ health-related quality of life.
References:
1Deodhar. Lancet 2020;395:1115-25
2Mease. Lancet 2020;395:1126-36
3Mease. 6-M Persistence and Multi-Domain Effectiveness of GUS in Adults with PsA: RW Data from the CorEvitas Registry [abstr]. Maui Derm; June 21-24, 2023; Colorado Springs, CO
To cite this abstract in AMA style:
Mease P, Ogdie A, Tesser J, shiff N, Lin I, Chakravarty S, Kelleman M, Dodge R, McLean R, Broadwell A, Kavanaugh A, Merola J. Improvements in Patient-Reported Outcomes Through 6 Months of Guselkumab Treatment in Patients with Active Psoriatic Arthritis: Real-World Data from the CorEvitas Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/improvements-in-patient-reported-outcomes-through-6-months-of-guselkumab-treatment-in-patients-with-active-psoriatic-arthritis-real-world-data-from-the-corevitas-psoriatic-arthritis-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-patient-reported-outcomes-through-6-months-of-guselkumab-treatment-in-patients-with-active-psoriatic-arthritis-real-world-data-from-the-corevitas-psoriatic-arthritis-spondyloarthritis/