Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
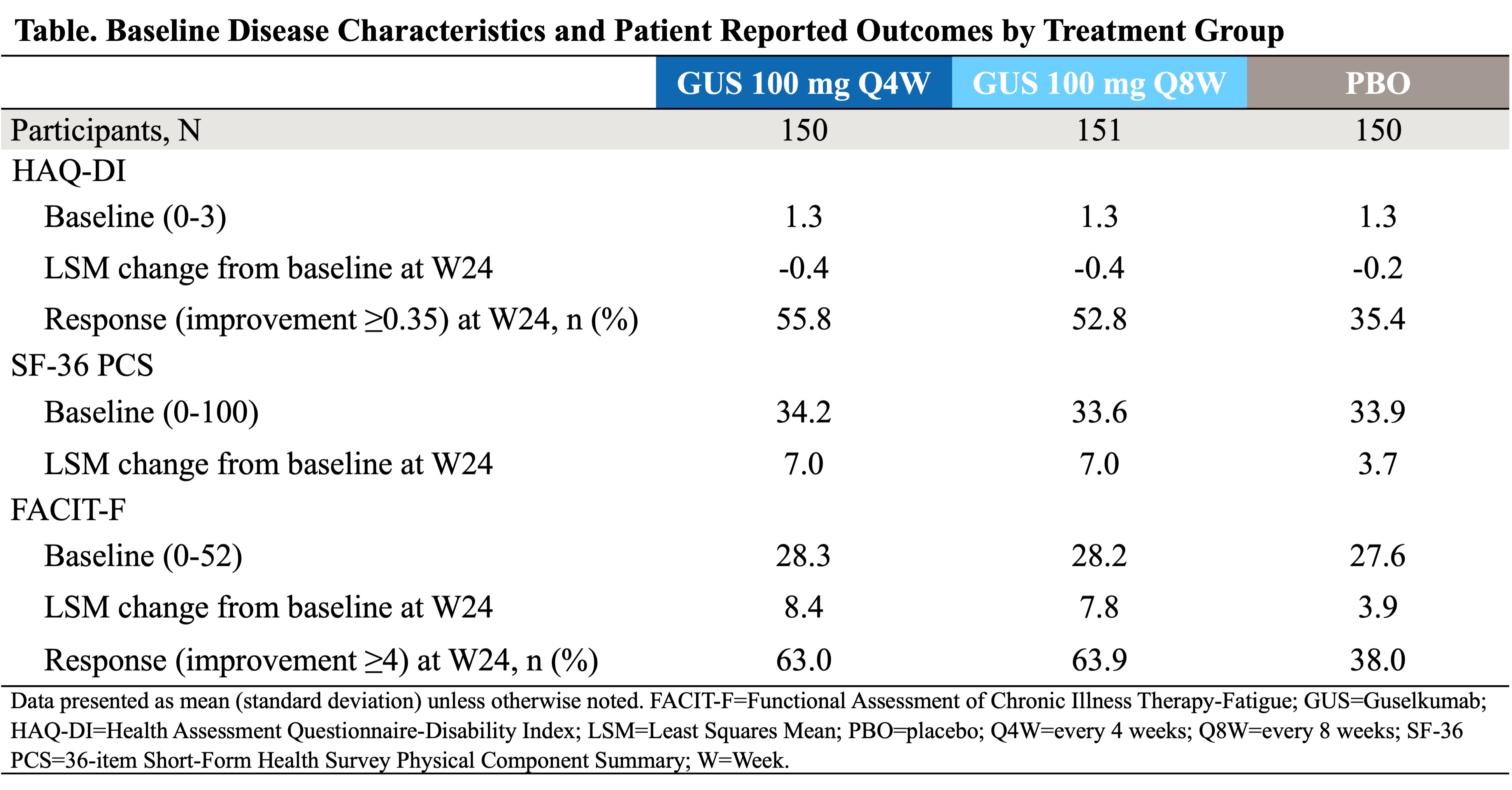
Background/Purpose: Guselkumab (GUS), a fully human IL-23p19-subunit inhibitor, has demonstrated efficacy in significantly improving psoriatic arthritis (PsA) signs and symptoms in participants (pts) with active PsA irrespective of prior tumor necrosis factor inhibitor (TNFi) experience. SOLSTICE is an ongoing Phase 3b, multicenter, randomized, double-blinded placebo-controlled study designed to evaluate the efficacy and safety of GUS on signs and symptoms of PsA disease specific patient reported outcomes (PROs), with two maintenance dosing regimens of 100 mg every 4 weeks (Q4W) and Q8W, in a dedicated PsA pt population who were inadequate responders (IR [inadequate efficacy and/or intolerance]) to one prior TNFi.
Methods: Adults (≥18 years) with active PsA (≥3 swollen joints; ≥3 tender joints; C-reactive protein [CRP] ≥0.3 mg/dL) who were TNFi-IR to one prior TNFi were eligible to be enrolled from 128 sites across 12 countries. Pts were randomized 1:1:1 to GUS 100 mg Q4W; GUS at Week (W)0, W4, then Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24. The major secondary PRO endpoints at W24 were mean change from baseline in Health Assessment Questionnaire-Disability Index (HAQ-DI; physical function), 36-item Short-Form Health Survey Physical Component Summary (SF-36 PCS; overall Health-related Quality of Life [HRQoL]), and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT- F) scores. Proportions of pts achieving clinically meaningful improvements for HAQ-DI response (improvement ≥0.35) and FACIT-F response (improvement ≥4) were prespecified endpoints, but not multiplicity-controlled.
Results: A total of 451 participants (Q4W n=150, Q8W n=151, PBO n=150) were included in the analysis. On average, pts had moderate to severe impairment of physical function at baseline that is consistent with an active PsA population (Table). At W24, greater improvements in HAQ-DI, SF-36 PCS, and FACIT-F scores were observed in both treatment groups vs the PBO group (Table). A significantly greater reduction from baseline in HAQ-DI score was observed at W24 in the Q4W (-0.42, p=0.003) and Q8W (-0.40, p=0.008) groups, vs PBO (-0.24) group (Figure 1a). Furthermore, significantly greater improvement from baseline was observed in the Q4W and Q8W groups vs PBO group for SF-36 PCS (7.0 and 7.0 vs 3.7, respectively) and FACIT-F (8.4 and 7.8 vs 3.9, respectively); p< 0.001 for both (Figure 1b). Among pts with a baseline score of ≥0.35, the proportion of pts who achieved HAQ-DI response at W24 in the Q4W and Q8W groups vs PBO group were, 55.8% (p< 0.001) and 52.8% (p=0.003) vs 35.4%, respectively (Figure 2a). Greater proportions of pts achieved clinically meaningful improvement in FACIT-F response in the Q4W (63.0%) and Q8W (63.9%) groups (p< 0.001 for both) vs PBO (38.0%) group (Figure 2b).
Conclusion: In the TNFi-IR PsA population from SOLSTICE, both GUS dosing regimens demonstrated superior efficacy vs PBO for improving physical function, overall HRQoL, and fatigue at W24. These data reinforce the effectiveness of GUS irrespective of dosing regimen in the TNFi-IR population across multi-domain measures of efficacy and PROs.
To cite this abstract in AMA style:
Gottlieb A, Merola J, Mease P, Ritchlin C, Scher J, Parnell Lafferty K, Chan D, Chakravarty S, Langholff W, Wang Y, Choi, MD, PhD, FAAD O, Krol Y, Ogdie A. Improvements in Patient Reported Outcomes Through 24 Weeks of Guselkumab Treatment in Participants with Active Psoriatic Arthritis and Inadequate Response and/or Intolerance to One Prior Tumor Necrosis Factor Inhibitor [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/improvements-in-patient-reported-outcomes-through-24-weeks-of-guselkumab-treatment-in-participants-with-active-psoriatic-arthritis-and-inadequate-response-and-or-intolerance-to-one-prior-tumor-necrosi/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-patient-reported-outcomes-through-24-weeks-of-guselkumab-treatment-in-participants-with-active-psoriatic-arthritis-and-inadequate-response-and-or-intolerance-to-one-prior-tumor-necrosi/

.jpg)
.jpg)