Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Secukinumab has shown to be well tolerated and effective in active ankylosing spondylitis (AS) patients in a proof-of-concept trial1. Briefly, at Week (Wk) 6, 61% (14/23) patients on secukinumab achieved Assessment of SpondyloArthritis International Society (ASAS) 20 response vs 17% (1/6) patients on placebo and the primary endpoint was met. Here we explored the modulation of markers of inflammation following secukinumab treatment, including CRP, ESR and S100 proteins A8 and A9 (calgranulin A and B), recently postulated to play a role as markers of inflammation in inflammatory arthritis2.
Methods: 30 patients were randomized 4:1 to either secukinumab 2×10 mg/kg or placebo (i.v. infusion, 3 wks apart). Primary endpoint was the proportion of patients achieving ASAS 20 response at Wk 6. Key secondary endpoints included safety and tolerability and ASAS responses up to Wk 24. S100 calgranulins were analyzed pre- and post-treatment using an in-house validated multiplex assay and high sensitivity (hs) CRP assay. In accordance with the protocol, descriptive statistics were applied for exploratory analyses, due to the small sample size in the placebo arm. No group comparisons were conducted between secukinumab and placebo.
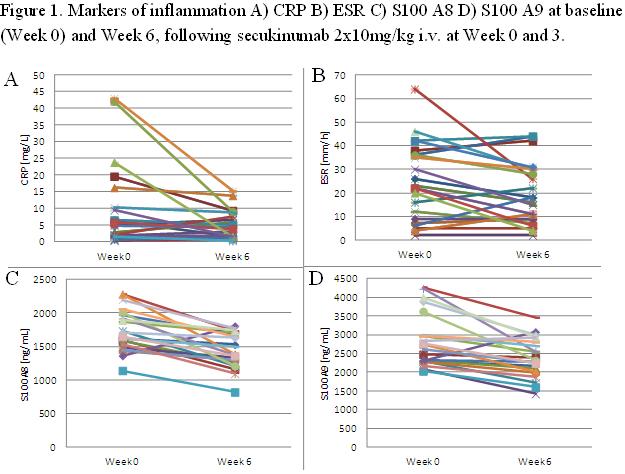
Results: Measurements from pre-treatment (Wk 0) and post-treatment (Wk 6) were available from 21 (CRP) and 14 secukinumab patients (S100A8/9), respectively. Baseline mean (SD) CRP values were 13.84 mg/L (17.40) for CRP; 1819 (717) for S100A8; and 2013 (835) ng/mL for S100A9, respectively. Baseline elevations of CRP (> normal upper limit of 10 mg/L) were observed for only a minority of patients on secukinumab (N=8). Comparing Wk 0 with Wk 6, significant changes were seen for CRP (-19%, p=0.027, n=21), S100A8 (-25%, p=0.012, n=14) and S100A9 (-21%, p=0.008, n=14) (Figure 1). Baseline levels of CRP neither correlated with Wk 6 ASAS20 nor ASAS40 responses (R=0.03 for both). Likewise, DCRP (Wk 0-6) neither correlated with Wk 6 ASAS20 (R=0.13) nor ASAS40 (R=0.15). In contrast, correlations were seen for S100A8 and A9 baseline concentrations with ASAS20 (R=0.55 and 0.39, respectively), and ASAS40 (R=0.40 and 0.59, respectively). Moreover, DS100A8 (Wk 0-6) was correlated with Wk 6 ASAS20 (R=0.43) and ASAS40 (R=0.52); and DS100A9 (Wk 0-6) with Wk 6 ASAS20 (R=0.28) and ASAS40 (R=0.64).
Conclusion: In this trial of secukinumab in AS, exploratory analyses of selected inflammatory markers suggest that secukinumab reduces CRP, S100A8 and S100A9, but only S100A8/9 reductions appear to correlate with clinical responses at Wk 6. Lack of correlation of CRP reductions with Wk 6 ASAS response may be attributable to the low number of patients with baseline CRP elevations. Further studies of S100 proteins in AS and their relationship with IL-17A blockade are warranted.
References:
1. Baeten et al, Ann Rheum Dis. 2011;70:127.
2. Kruithof et al, Arthritis Rheum. 2006;54:1795-804.
Disclosure: D. L. Baeten,
Abbott Immunology Pharmaceuticals; Pfizer Inc; Centocor, Inc, 2,
2;
S. Bek,
Novartis Institutes for Biomedical Research,
2;
J. Wei,
Pharma Development IIS,
3;
A. Brachat,
Novartis Institutes for Biomedical Research,
3;
J. Sieper,
None;
P. Emery,
None;
J. Braun,
None;
D. M. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth ,
2;
I. B. McInnes,
Novartis Pharmaceutical Corporation,
2;
J. M. van Laar,
None;
R. Landewe,
Abbott, Amgen, AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB, Wyeth Rheumatology Consultancy BV ,
2;
P. Wordsworth,
Abbott Laboratories; Merck Pharmaceuticals ,
8;
J. Wollenhaupt,
None,
9;
H. Kellner,
None,
9;
J. E. Paramarta,
None,
9;
A. Bertolino,
Novartis Institute of Biomedical Research,
3;
A. Wright,
Novartis Pharma AG,
3;
H. Wolfgang,
Novartis Institute of Biomedical Research,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-signs-and-symptoms-of-active-ankylosing-spondylitis-following-treatment-with-anti-interleukin-il-17a-monoclonal-antibody-secukinumab-are-paralleled-by-reductions-in-acute-phase-marker/