Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
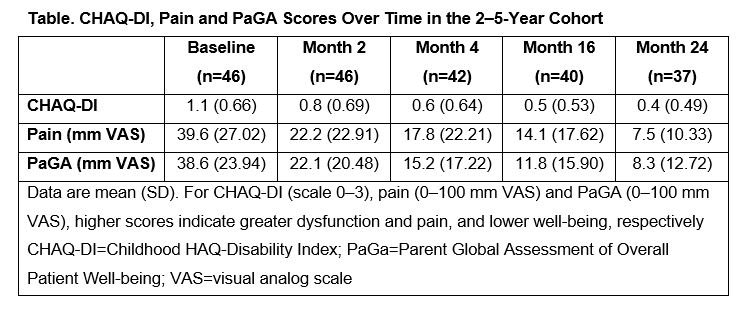
Background/Purpose: Efficacy of SC abatacept in patients with polyarticular-course JIA (pJIA) was shown in a 2-year, open-label, Phase III international study (NCT01844518).1 Pediatric patient-reported outcomes (PROs) were improved with IV and SC abatacept in patients aged 6–17 years.2,3 Here we report the effect of SC abatacept on PROs (Activity Limitation Questionnaire [ALQ], Childhood HAQ-Disability Index [CHAQ-DI], Pain Assessment and Parent Global Assessment of Overall Patient Well-being [PaGA]) in the 2–5-year-old patients with active pJIA over 2 years.
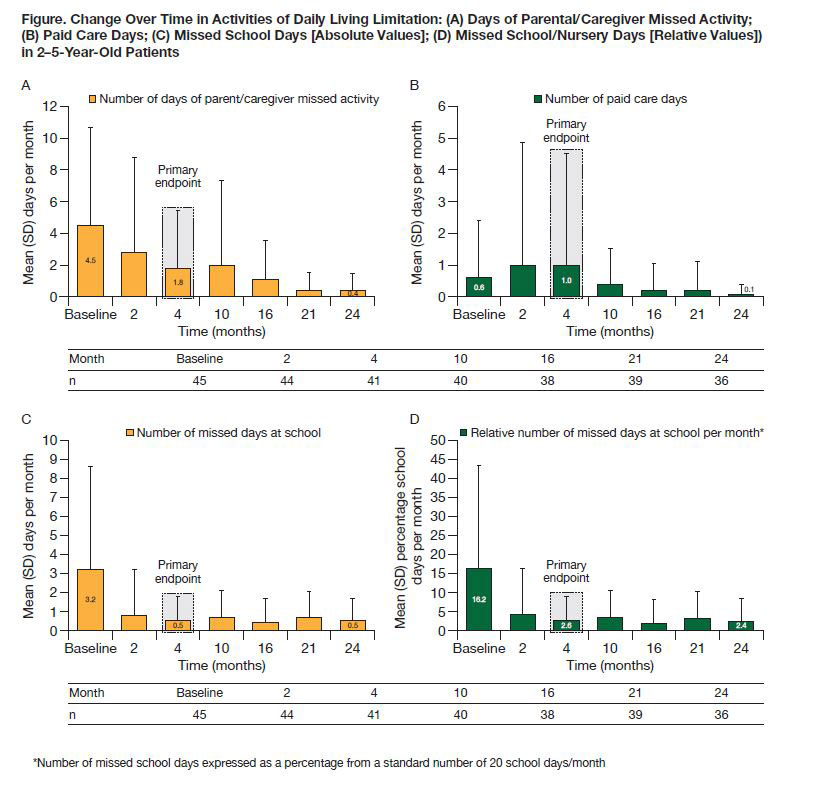
Methods: Patients with pJIA were grouped into two age cohorts (2–5 and 6–17 years) and received weight-tiered SC abatacept (10 to < 25 kg [50 mg], 25 to < 50 kg [87.5 mg], ≥50 kg [125 mg]) weekly for 4 months. JIA-ACR30 criteria responders at Month 4 could receive SC abatacept for another 20 months.1 For the 2–5-year cohort reported here, ALQ (mean [SD] number of days of parental/caregiver missed activity, paid care and missed school [absolute values per month and percentage of days missed per month relative to an assumed average of 20 school days/month]), CHAQ-DI (0–3 scale across 8 domains of disability component), pain (0–100 mm visual analog scale [VAS]) and PaGA (0–100 mm VAS) were evaluated.
Results: Baseline characteristics of the 46 patients with pJIA from the 2–5-year cohort included median (min, max) age of 4.0 (2.0, 5.0) years and median (interquartile range) number of active joints of 7.0 (6.0, 12.0). At baseline, 80.4% of patients received MTX (median dose: 13.3 mg/m2/week) and 21.7% of patients had prior biologic failure. CHAQ-DI, pain and PaGA improved from baseline to Month 24 (Table). Most ALQ components improved from baseline to Month 4 (primary endpoint); these improvements were largely maintained over time to Month 24 (Figure). Relative percentage of days missed from school decreased from 16.2% (baseline) to 2.4% (Month 24, Figure D).
Conclusion: In this analysis of patients with pJIA aged 2–5 years, SC abatacept demonstrated a beneficial effect on PROs including improvement in well-being (PaGA) and reductions in disability (CHAQ-DI), pain and activity limitation (ALQ) over 24 months.
References:
- Brunner HI, et al. Arthritis Rheumatol 2018;70:1144–54.
- Ruperto N, et al. Arthritis Care Res (Hoboken) 2010;62:1542–51.
- Ruperto N, et al. Ann Rheum Dis 2017;76(suppl 2):75.
Professional medical writing assistance was provided by Katerina Kumpan, PhD, at Caudex, funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Brunner H, Tzaribachev N, Louw I, Antón J, Viola D, Lauwerys B, Cuttica R, Quartier P, Gervais E, Belot A, Minden K, Lutz T, Cimaz R, Ally M, van Zyl R, Calvo Penadés I, Zhuo J, Wong R, Nys M, Elbez Y, Martini A, Lovell D, Ruperto N. Improvement in Patient-Reported Outcomes in Patients Aged 2–5 Years with Polyarticular-Course JIA Treated with Subcutaneous Abatacept: 2-Year Results from a Phase III International Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improvement-in-patient-reported-outcomes-in-patients-aged-2-5-years-with-polyarticular-course-jia-treated-with-subcutaneous-abatacept-2-year-results-from-a-phase-iii-international-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-patient-reported-outcomes-in-patients-aged-2-5-years-with-polyarticular-course-jia-treated-with-subcutaneous-abatacept-2-year-results-from-a-phase-iii-international-study/