Session Information
Date: Monday, November 8, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster II (1364–1390)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic sclerosis (SSc) is a chronic disease characterized by vasculopathy, inflammation and fibrosis. Rheumatologists have limited options to effectively treat rapidly progressive disease. There is an important unmet medical need for disease modifying therapy for patients with SSc. Autologous hematopoietic stem cell transplantation (AHSCT) has been shown in randomized controlled trials and is well recognized to be an effective treatment for rapidly progressive SSc. However, there is a paucity of data pertaining to its performance as compared to real-world routine clinical practice. The objective of this study was to evaluate the effectiveness of AHSCT for SSc compared to conventional care used in routine clinical practice.
Methods: SSc patients from France who underwent AHSCT were compared to SSc patients who met criteria for AHSCT (as defined in the ASTIS trial1) but received conventional care in Canada. The primary outcome was overall survival. Secondary outcomes included modified Rodnan skin score (mRSS), forced vital capacity (FVC) and diffusion capacity for carbon monoxide (DLCO). Baseline characteristics were compared using descriptive statistics. Overall survival for both groups was estimated by constructing Kaplan-Meier survival curves based on time to death. Measures of mRSS, FVC and DLCO were compared using linear regression models. Analyses were adjusted for baseline scores and incorporated stabilized inverse probability of treatment weights to account for confounding by indication. Propensity scores were estimated using logistic regression.
Results: 41 SSc patients who underwent AHSCT and 85 patients treated with conventional care were compared. Baseline characteristics are delineated in Table 1. Mean mRSS was 25.0 (10.5) in the AHSCT group and 27.0 (8.0) in the conventional care group. Mean FVC and DLCO were 78.9 (17.5) and 55.2 (15.5) in the AHSCT group and 79.0 (20.2) and 62.0 (19.6) in the conventional care group, respectively. AHSCT was associated with improvement in overall survival (log-rank p=0.115; Figure 1). In follow-up, the mRSS was lower with AHSCT compared to conventional care: 7.25 point between group difference at 12 months (p=< 0.001), 6.41 points at 24 months (p=< 0.001) and 4.48 points at 36 months (p=< 0.001). There was no statistically significant difference in FVC between groups at 12 months but at 24 months, AHSCT was associated with a higher FVC (between group difference of 9.22 (p=< 0.001)) but a lower DLCO (between group difference of -3.43 (p=0.002)).
Conclusion: The present study provides crucial real-world long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with SSc.
1. Van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA J. Am. Med. Assoc. 311, 2490-2498 (2014).
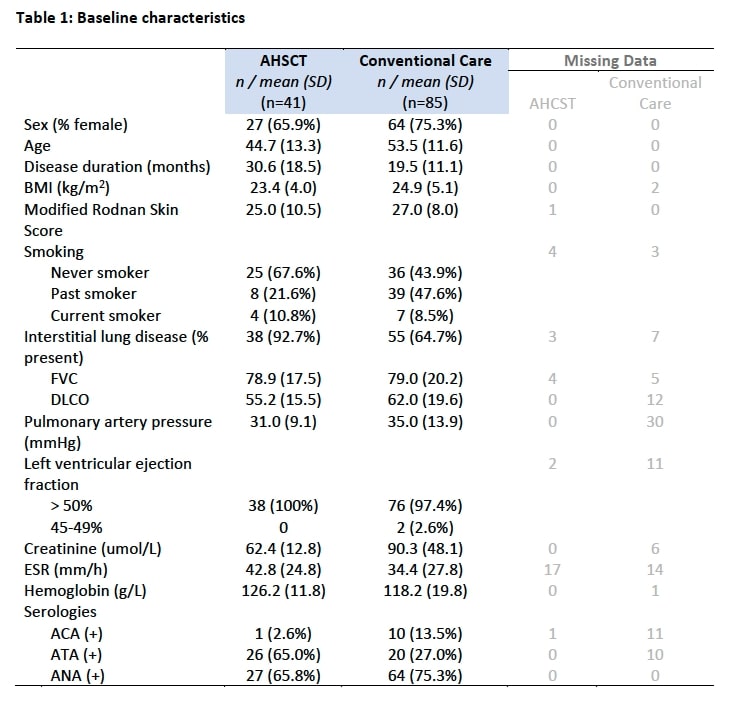 Table 1: Baseline characteristics
Table 1: Baseline characteristics
 Figure 1: Kaplan-Meier survival estimates of overall survival adjusted for stabilized inverse probability of treatment weights
Figure 1: Kaplan-Meier survival estimates of overall survival adjusted for stabilized inverse probability of treatment weights
To cite this abstract in AMA style:
Maltez N, Wang M, Wells G, Tugwell P, Baron M, Marjanovic Z, Lansiaux P, Farge D, Hudson M. Improvement in Overall Survival, Skin Fibrosis and Lung Function with Autologous Hematopoietic Stem Cell Transplantation in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improvement-in-overall-survival-skin-fibrosis-and-lung-function-with-autologous-hematopoietic-stem-cell-transplantation-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-overall-survival-skin-fibrosis-and-lung-function-with-autologous-hematopoietic-stem-cell-transplantation-in-systemic-sclerosis/
