Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Japanese post-marketing surveillance (PMS) data demonstrated that effectiveness of abatacept in rheumatoid arthritis (RA) patients with previous biologics treatment (bio-switch) was significantly lower than that in bio-naïve patients. Useful predictive factors for good clinical response of abatacept is necessary especially in the bio-switch patients. Serum matrix metalloproteinase-3 (MMP-3) is an enzyme produced by synoviocytes which can be used to predict clinical effectiveness and joint destruction in RA patients. However, little is known about the relationship between MMP-3 and effectiveness of abatacept. This study aimed to study whether serum MMP-3 levels can predict good clinical effectiveness of abatacept in the bio-switch RA patients using data from a multicenter cohort.
Methods: Participants were consecutive 423 RA patients treated with abatacept, observed for longer than 52 weeks, and registered in the TBCR, a Japanese multicenter registry system for RA patients treated with biologics. Multivariate logistic regression analysis was used to study predictive factors for achievement of low disease activity at 52 weeks separately in bio-naïve and bio-switch group.
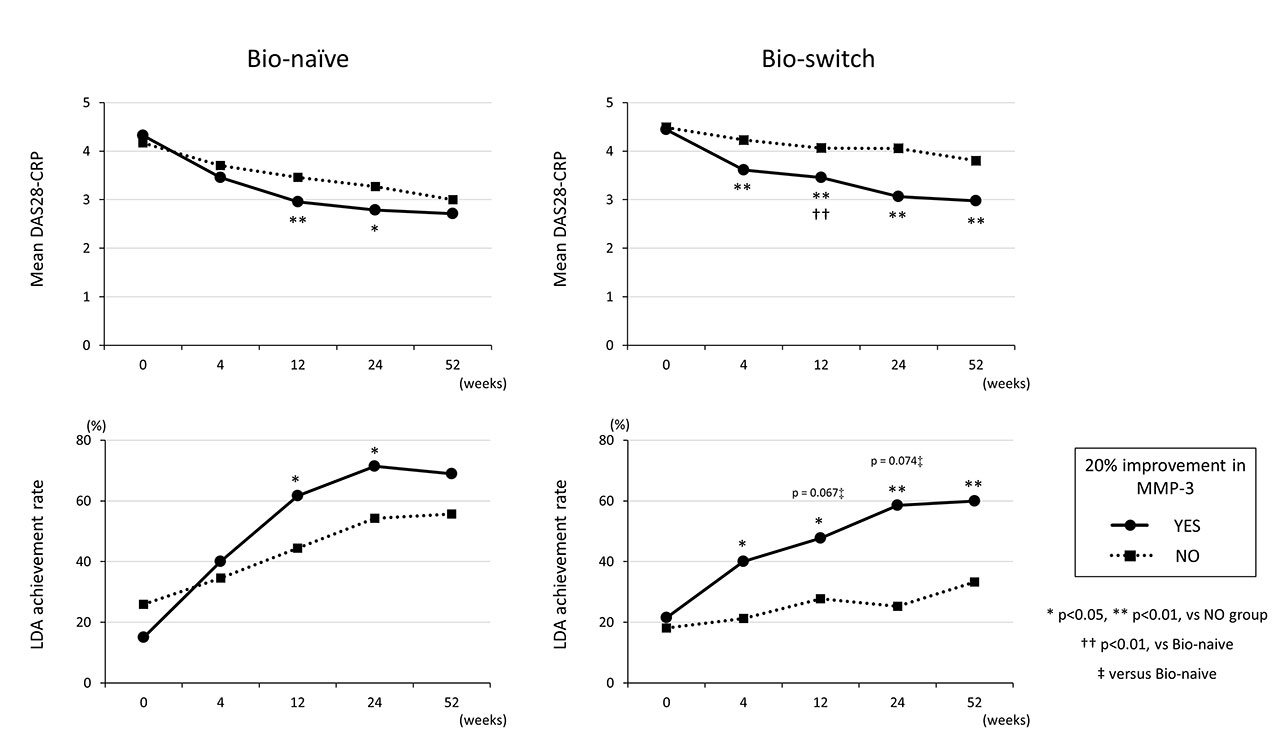
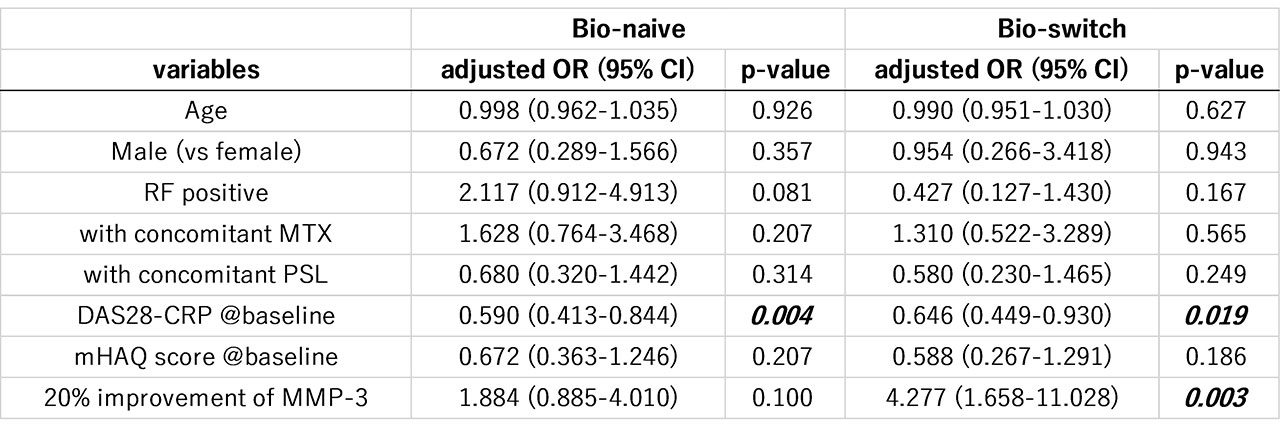
Results: A total of 189 bio-switch and 234 bio-naïve patients were included in this study. ROC analysis revealed that MMP-3 improvement rate at 12 weeks, compared to 4 and 24 weeks, had highest AUC and the best cut-off value was 20.0% at 12 weeks for predicting achievement of low disease activity (LDA) at 52 weeks in the bio-switch group (Figure 1, right panel). We performed multivariate logistic regression analysis to study independent predictive factors for LDA at 52 weeks using patients‘ background factors as well as 20% improvement of MMP-3 at 12 weeks. In the bio-naïve group, DAS28-CRP score at baseline was the only predictive factor, while the achievement of 20% improvement of MMP-3 at 12 weeks was an independent predictive factor (adjusted OR: 3.550, p=0.005) in addition to DAS28-CRP in the bio-switch group (Table). In the bio-switch group, patients that achieved 20% improvement of MMP-3 at 12 weeks demonstrated significantly higher achievement rate of LDA at 52 weeks compared to those that did not achieved 20% improvement (60.0 vs 33.3%, p=0.001) (Figure 2).
Conclusion: In the bio-switch patients, we sometimes have difficulty to obtain good clinical response of abatacept and it would be even more important to judge whether to continue abatacept or whether to add other anti-rheumatic drugs as early as possible.
Our results suggest that improvement in MMP-3 levels at 12 weeks is key to predicting the clinical efficacy of abatacept at 1 year. It will be important to pay closer attention not only to major clinical indices such as DAS28, but also to changes in MMP-3 levels, in order to optimize clinical outcomes when treating Bio-switch patients with abatacept.
To cite this abstract in AMA style:
Takahashi N, Kojima T, Terabe K, Asai S, Ishiguro N. Improvement in Matrix metalloproteinase-3 Levels at 12 Weeks Independently Predicts Achievement of Low Disease Activity at 52 Weeks in Bio-switch Patients with Rheumatoid Arthritis Treated with Abatacept [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improvement-in-matrix-metalloproteinase-3-levels-at-12-weeks-independently-predicts-achievement-of-low-disease-activity-at-52-weeks-in-bio-switch-patients-with-rheumatoid-arthritis-treated-with-abatac/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-matrix-metalloproteinase-3-levels-at-12-weeks-independently-predicts-achievement-of-low-disease-activity-at-52-weeks-in-bio-switch-patients-with-rheumatoid-arthritis-treated-with-abatac/