Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
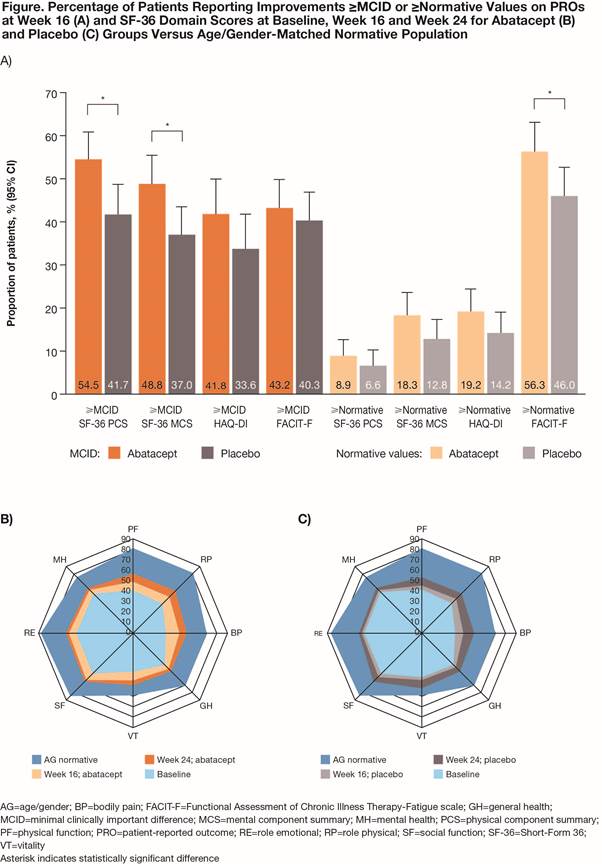
Conclusion: Abatacept treatment improved many PROs in pts with active PsA, with larger benefits in the elevated CRP subpopulation and regardless of prior TNFi exposure.
Disclosure: V. Strand, AbbVie, Amgen Corporation, AstraZeneca, Biogen, BMS, Boehringer Ingelheim, Celgene, Celltrion, Corrona, Crescendo / Myriad Genetic, EMD Serono, Genentech / Roche, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sano, 5; E. Alemao, Bristol-Myers Squibb, 1,Bristol-Myers Squibb, 3; T. Lehman, Bristol-Myers Squibb, 3,Bristol-Myers Squibb, 1, 9; A. Johnsen, Bristol-Myers Squibb, 1,Bristol-Myers Squibb, 3; S. Banerjee, Bristol-Myers Squibb, 3,Bristol-Myers Squibb, 1, 9; H. Ahmad, Bristol-Myers Squibb, 3,Bristol-Myers Squibb, 1, 9; P. J. Mease, AbbVie, Amgen, BMS, Celgene, Janssen, Lilly, Novartis, Pfizer, Sun, UCB, 2,AbbVie, Amgen, BMS, Celgene, Crescendo Bioscience, Corrona, Demira, Janssen, Lilly, Novartis, Pfizer, Sun, UCB, Zynerba, Speaker Bureau: AbbVie, Amgen, BMS, Celgene, Crescendo Bioscience, Genentech, Janssen, Novartis, Pfizer, UCB, 5.
To cite this abstract in AMA style:
Strand V, Alemao E, Lehman T, Johnsen A, Banerjee S, Ahmad H, Mease PJ. Improved Patient-Reported Outcomes in Psoriatic Arthritis Patients Treated with Abatacept: Results from a Phase III Trial [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/improved-patient-reported-outcomes-in-psoriatic-arthritis-patients-treated-with-abatacept-results-from-a-phase-iii-trial/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improved-patient-reported-outcomes-in-psoriatic-arthritis-patients-treated-with-abatacept-results-from-a-phase-iii-trial/