Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is an autoimmune disorder characterized by progressive inflammation and irreversible joint damage. Treatment of active disease includes disease-modifying anti-rheumatic drugs (DMARDs) and short-term corticosteroid (CS) use. A comprehensive assessment of clinical treatment outcomes should include validated patient-reported outcome (PRO) instruments. Repository corticotropin injection (RCI) is approved by the FDA for short-term adjunctive use in the treatment of RA.1 A naturally sourced complex mixture of purified adrenocorticotropic hormone (ACTH1-39) analogues and other pituitary peptides, RCI stimulates endogenous CS production and is an agonist for all 5 melanocortin receptors (MCRs).1,2 MCR activation by ACTH has been shown to have direct and indirect anti-inflammatory and immunomodulatory effects.2 A 2-part, multicenter, placebo-controlled Phase 4 trial explored the effect of RCI treatment on PROs with the greatest impact on quality of life (ie, for pain, fatigue, physical functioning, and ability to work) in patients with persistently active RA. Data presented are from the open-label period (ClinicalTrials.gov ID: NCT02919761).
Methods: Adults with persistently active RA (defined as DAS28-ESR >3.2) despite DMARD and CS use received 80 U of RCI subcutaneously, twice weekly during a 12-week open-label period. PRO measurements included the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scale, the Work Productivity and Activity Impairment (WPAI) questionnaire, and the Health Assessment Questionnaire – Disability Index (HAQ-DI). Mean changes from baseline were assessed at Weeks 4, 8, and 12. Patients’ global assessments of disease activity and pain were also evaluated.
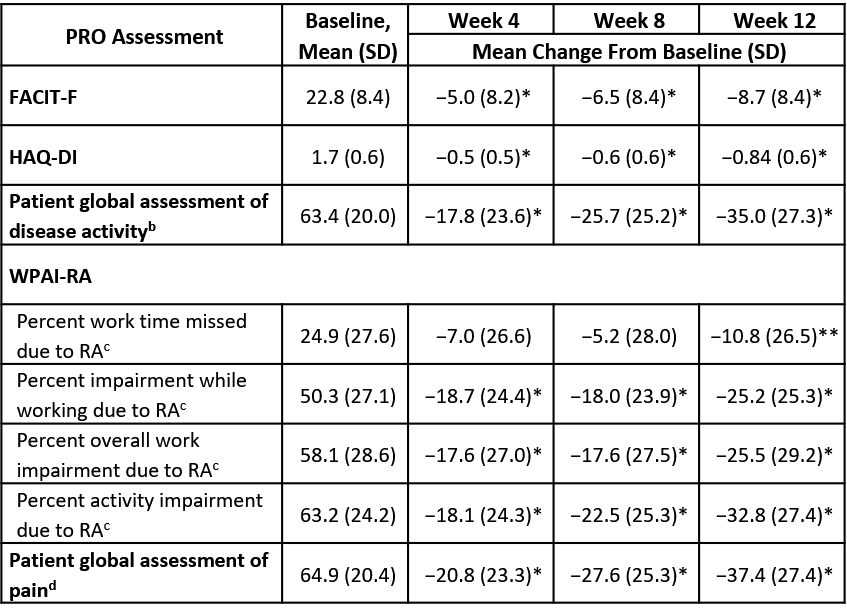
Results: Of the 259 patients in the modified intent-to-treat population, 89.2% were female and 65.6% were Caucasian (mean age, 51 years). During the 12-week open-label treatment period (wherein 62.9% of patients [163/259] achieved low disease activity by Week 12), mean improvements from baseline were clinically and statistically significant at all time points for HAQ-DI and FACIT-F scores. Clinically and statistically significant improvements in 3 of 4 WPAI subscores – percent impairment while working, percent activity impairment, and percent overall work impairment – were observed at all time points; for percent work time missed, significant improvement was achieved by Week 12. Clinically and statistically significant improvements from baseline at Weeks 4, 8, and 12 were found for patient-reported global assessments of disease activity and pain (see Table for results).
Conclusion: During the 12-week open-label treatment period, RCI significantly improved patient-reported pain, fatigue, physical functioning, and work-related impairment as early as Week 4. RCI treatment resulted in rapid and clinically meaningful improvements in PROs deemed most relevant to quality of life by patients with persistently active RA.
- Acthar® Gel (repository corticotropin injection) [prescribing information]. Mallinckrodt ARD, LLC, 2019.
- Catania A, et al. ScientificWorldJournal. 2010;10:1840-53.
a mITT population -all patients who received study drug and had any post-treatment efficacy assessment-.
b MCID = 15% absolute/20% relative improvement.2
c MCID = 7% absolute change.3
d MCID = 11.4
Abbreviations and MCID references: FACIT-F; Functional Assessment of Chronic Illness Therapy – Fatigue -MCID = 3-41-; HAQ-DI, Health Assessment Questionnaire – Disability Index -MCID = 0.21-; MCID, minimum clinically important difference; mITT, modified intent-to-treat; PRO, patient-reported outcome; RCI, repository corticotropin injection; SD, standard deviation; WPAI-RA, Work Productivity and Activity Impairment Questionnaire – Rheumatoid Arthritis.
1 Wells GA, et al. J Rheumatol. 1993;20-2-:557-60. 2 Tubach F, et al. Arthritis Care Res -Hoboken-. 2012;64-11-:1699-1707. 3 Reilly MC, et al. Gut. 2007;56-Suppl 3-:159. 4 Hawker GA, et al. Arthritis Care Res. 2011;63-Suppl 11-:S240-52.
To cite this abstract in AMA style:
Furst D, Wan G, Liu J, Zhu J, Bartels-Peculis L, Panaccio M, Fleischmann R. Improved Patient-Reported Outcomes in Patients with Persistently Active Rheumatoid Arthritis Following Treatment with Repository Corticotropin Injection [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improved-patient-reported-outcomes-in-patients-with-persistently-active-rheumatoid-arthritis-following-treatment-with-repository-corticotropin-injection/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improved-patient-reported-outcomes-in-patients-with-persistently-active-rheumatoid-arthritis-following-treatment-with-repository-corticotropin-injection/